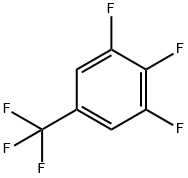
3,4,5-TRIFLUOROBENZOTRIFLUORIDE synthesis
- Product Name:3,4,5-TRIFLUOROBENZOTRIFLUORIDE
- CAS Number:80172-04-9
- Molecular formula:C7H2F6
- Molecular Weight:200.08
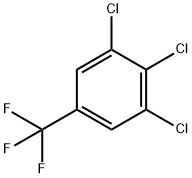
50594-82-6

80172-04-9
The general procedure for the synthesis of 3,4,5-trichlorobenzotrifluoride from 3,4,5-trichlorobenzotrifluoride was as follows: 860 g of dried cyclobutanesulfone and 524 g of dried potassium fluoride (KF) were added to an autoclave followed by the addition of an appropriate amount of water. Next, 500 g of 3,4,5-trichlorobenzotrifluoride, 5 g of nitrobenzene and 25 g of CNC catalyst were added. After sealing the autoclave, the reaction mixture was heated to 200 °C and maintained for 5 hours, then warmed up to 220 °C to continue the reaction for 12 hours. The maximum total pressure was controlled at 9 bar during the reaction. Upon completion of the reaction, the mixture was cooled to 20 °C and the product was transferred to the cooled receiver by depressurization. Subsequently, the product was distilled at standard pressure. The crude product was redistilled to give 300 g of 3,4,5-trifluorobenzotrifluoride in 75% of the theoretical value and the product was a colorless liquid.

50594-82-6
244 suppliers
$6.00/5g

80172-04-9
98 suppliers
$29.00/1g
Yield: 75%
Reaction Conditions:
with potassium fluoride;nitrobenzene;1,3-dimethyl-2-(N',N',N",N"-tetramethylguanidino)-4,5-dihydro-3H-imidazolium chloride in sulfolane at 200 - 220; under 6750.68 Torr; for 17 h;
Steps:
3
Example 3; Preparation method of 3,4,5-trifluorobenzotrifluoride; 860 g of dry sulpholane and 524 g of dry KF were initially charged with exclusion of moisture in an autoclave, and 500 g of 3,4,5-trichlorobenzotrifluoride, 5 g of nitrobenzene and 25 g of CNC catalyst were added. The vessel was sealed and the mixture subsequently heated to 200° C. for 5 h and then to 220° C. for a further 12 h. During the reaction, a maximum total pressure of 9 bar arose. The mixture was then cooled to 20° C. and decompressed into a cooled receiver. The product was distilled off at standard pressure. After redistillation of the crude product, 300 g of 3,4,5-trifluorobenzotrifluoride (75% of theory) were obtained as a colourless liquid.
References:
Pleschke, Axel;Marhold, Albrecht US2006/9643, 2006, A1 Location in patent:Page/Page column 5
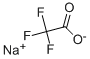
2923-18-4
295 suppliers
$5.00/10g
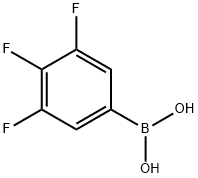
143418-49-9
411 suppliers
$5.00/1g

80172-04-9
98 suppliers
$29.00/1g