
3-(4-BROMO-BENZOYL)-PYRROLIDIN-2-ONE synthesis
- Product Name:3-(4-BROMO-BENZOYL)-PYRROLIDIN-2-ONE
- CAS Number:328546-97-0
- Molecular formula:C11H10BrNO2
- Molecular Weight:268.11
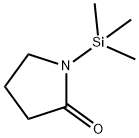
14468-90-7
85 suppliers
$49.20/10g
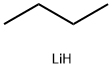
29786-93-4
0 suppliers
inquiry
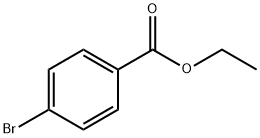
5798-75-4
204 suppliers
$6.00/5g

328546-97-0
14 suppliers
inquiry
Yield:328546-97-0 4.08 g (71%)
Reaction Conditions:
with acetic acid;diisopropylamine in tetrahydrofuran;ethyl acetate;
Steps:
148.a (a)
(a) 3-[1-(4-Bromo-phenyl)-methanoyl]pyrrolidin-2-one: Diisopropylamine (3.75 mL, 26.73 mmol) was dissolved in THF (70 mL) and cooled to -78° C. n-Butyllithium (2.5M/hexanes, 10.69 mL, 26.73 mmol) was added dropwise, and the reaction stirred for 15 minutes at that temperature. 1-(Trimethylsilyl)-2-pyrrolidinone (Aldrich Chemical Co.) (4.28 mL, 25.67 mmol) was added dropwise and again stirred for 15 minutes at -78° C. Ethyl-4-bromobenzoate (5.00 g, 3.56 mL, 21.39 mmol) was added dropwise. The reaction was allowed to warm to rt and stirred overnight. The THF was removed in vacuo. The solids were redissolved in THF (70 mL) and 10% HOAc (40 mL). The THF was again removed and replaced with water. The product was extracted in EtOAc (3*). The organic phases were combined, washed with sat. NaHCO3, water, and brine, then dried (MgSO4). The product was purified by flash silica gel chromatography eluding with 0-2% MeOH/CHCl3 to give 4.08 g (71%) of a white solid: mp=167-169° C.; Rf=0.16 (2% MeOH/CHCl3); IR (KBr) 1699, 1587, 1397, 1273 cm-1; 1H NMR (DMSO-d6) δ 2.18-2.28 (m, 1H), 2.40-2.51 (m, 1H), 3.24-3.30 (m, 2H), 4.55-4.60 (m, 1H), 7.76 (d, 2H, J=8.7 Hz), 7.95-7.99 (m, 3H). LRMS 270 (M+H).
References:
US6548494,2003,B1