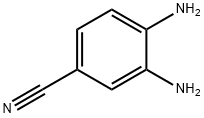
3,4-Diaminobenzonitrile synthesis
- Product Name:3,4-Diaminobenzonitrile
- CAS Number:17626-40-3
- Molecular formula:C7H7N3
- Molecular Weight:133.15
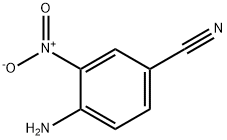
6393-40-4

17626-40-3
The general procedure for the synthesis of 3,4-diaminobenzonitrile from 4-amino-3-nitrobenzonitrile was as follows: palladium/carbon catalyst (500 mg, 10% purity) was added to a stirring solution of 4-amino-3-nitrobenzonitrile (2.00 g, 12.3 mmol) in methanol (20 mL). Subsequently, the reaction flask was degassed and filled with hydrogen (using a hydrogen balloon). The reaction mixture was stirred vigorously for 18 hours at 25°C. Upon completion of the reaction, the mixture was degassed under vacuum and backfilled with nitrogen, and this process was repeated three times. Afterwards, the reaction mixture was filtered through a diatomaceous earth pad and the filtrate was concentrated under vacuum to give 3,4-diaminobenzonitrile (1.47 g, 11.04 mmol, 90% yield) as a green oil, which was used directly in the next step of the reaction.
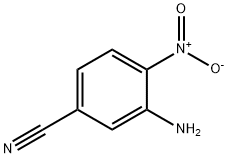
99512-10-4
52 suppliers
inquiry

17626-40-3
202 suppliers
$10.00/1g
Yield:17626-40-3 70%
Reaction Conditions:
with hydrogen;palladium-carbon in ethanol;
Steps:
101 3,4-diaminobenzonitrile
EXAMPLE 101 3,4-diaminobenzonitrile 3-Amino-4-nitrobenzonitrile (20.00 g, 0.042 mol) was hydrogenated under 50 psi of hydrogen gas in 250 mL of anhydrous ethanol with 1.00 g of 10% Pd/C for 5 hours. The mixture was then filtered through a pad of celite and the filtrate concentrated. Trituration of the resulting solid with diethyl ether afforded 11.40 g of the desired product as a tan solid (70%). 1H NMR (CD3OD): δ6.88 (m, 2H), 6.65 (d, 2H, J=8.53 Hz).
References:
US6221914,2001,B1

6393-40-4
197 suppliers
$15.00/1g

17626-40-3
202 suppliers
$10.00/1g
35704-19-9
120 suppliers
$12.00/5g

17626-40-3
202 suppliers
$10.00/1g

7732-18-5
507 suppliers
$13.50/100ML

6393-40-4
197 suppliers
$15.00/1g

17626-40-3
202 suppliers
$10.00/1g
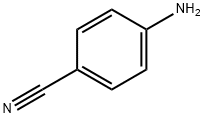
873-74-5
658 suppliers
$9.00/1g

17626-40-3
202 suppliers
$10.00/1g