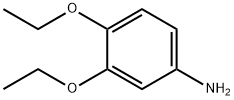
3,4-Diethoxyaniline synthesis
- Product Name:3,4-Diethoxyaniline
- CAS Number:39052-12-5
- Molecular formula:C10H15NO2
- Molecular Weight:181.23
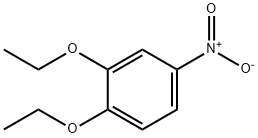
4992-63-6

39052-12-5
The general procedure for the synthesis of 3,4-diethoxyaniline from 3,4-diethoxynitrobenzene was as follows: 3,4-diethoxynitrobenzene (6.30 g, 29.9 mmol), 10% palladium-carbon catalyst (600 mg), and anhydrous ethanol (300 mL) were added to a reaction flask, and the reaction was stirred for 6 hours under hydrogen atmosphere. Upon completion of the reaction, the insoluble palladium-carbon catalyst was removed by filtration and the filtrate was concentrated under reduced pressure to afford 3,4-diethoxyaniline as an oily product in a yield of 5.02 g (92.9% yield). The product was characterized by 1H-NMR (CDCl3) with chemical shifts δ: 1.34 to 1.46 (6H, m), 3.94 to 4.08 (4H, m), 6.21 (1H, d, J = 2.8,8.4 Hz), 6.30 (1H, d, J = 2.6 Hz), 6.73 (1H, d, J = 8.4 Hz).

4992-63-6
48 suppliers
inquiry

39052-12-5
114 suppliers
$16.00/250mg
Yield: 92.9%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol for 6 h;
Steps:
68.2 (2)
3,4-Diethoxy aniline
A solution of 1, 2-ethoxy-4-nitrobenzene (6.30 g, 29.9 mmol), 10% palladium carbon (600 mg) and ethanol (300 ML) was stirred under hydrogen atmosphere for 6 hours.. The insolubles were filtered off, and the filtrate was concentrated, to obtain the titled compound as oil. 5.02 g (92.9%) 1H-NMR (CDCl3) δ; 1.34 to 1.46 (6H, m), 3.94 to 4.08 (4H, m), 6.21 (1H, d, J = 2.8, 8.4Hz), 6.30 (1H, d, J = 2.6 Hz), 6.73 (1H, d, J = 8.4 Hz)
References:
Takeda Chemical Industries, Ltd. EP1437344, 2004, A1 Location in patent:Page 37
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

39052-12-5
114 suppliers
$16.00/250mg
94-71-3
338 suppliers
$14.00/5g

39052-12-5
114 suppliers
$16.00/250mg
55483-70-0
3 suppliers
inquiry

39052-12-5
114 suppliers
$16.00/250mg
135325-75-6
0 suppliers
inquiry

39052-12-5
114 suppliers
$16.00/250mg