
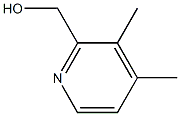
3,4-dimethyl-2-Pyridinecarboxaldehyde synthesis
- Product Name:3,4-dimethyl-2-Pyridinecarboxaldehyde
- CAS Number:780801-25-4
- Molecular formula:C8H9NO
- Molecular Weight:135.16
121638-25-3
2 suppliers
inquiry

780801-25-4
2 suppliers
inquiry
Yield:780801-25-4 80%
Reaction Conditions:
with manganese dioxide in dichloromethane at 20;
Steps:
56 EXAMPLE 56, COMPOUND 56: N1-(3,5-Dimethyl-pyridin-2-ylmethyl)-N1-(3,4-dimethyl-pyridin-2-ylmethyl)-butane-1,4-diamine (HBr salt)
EXAMPLE 56 COMPOUND 56: N1-(3,5-Dimethyl-pyridin-2-ylmethyl)-N1-(3,4-dimethyl-pyridin-2-ylmethyl)-butane-1,4-diamine (HBr salt) A CH2Cl2 solution (10 mL) of (3,4-dimethyl-pyridin-2-yl)-methanol (271 mg, 1.98 mmol) (Katz, R. B. et al. Synth. Commun. 1989, 19, 317-25) was treated with MnO2 (2.10 g, 21.8 mmol), and the resultant black suspension was stirred at room temperature overnight. The black suspension was filtered through a celite pad and the filtrate was concentrated in vacuo to afford 3,4-Dimethyl-pyridine-2-carbaldehyde (215 mg, 80%), without further purification, as a red oil. 1H NMR (CDCl3) δ 2.37 (s, 3H), 2.60 (s, 3H), 7.22-7.29 (m, 1H), 8.52 (d, 1H, J=4.3 Hz), 10.20 (s, 1H).
References:
US2004/209921,2004,A1 Location in patent:Page 36