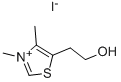
3,4-DIMETHYL-5-(2-HYDROXYETHYL)THIAZOLIUM IODIDE synthesis
- Product Name:3,4-DIMETHYL-5-(2-HYDROXYETHYL)THIAZOLIUM IODIDE
- CAS Number:16311-69-6
- Molecular formula:C7H12INOS
- Molecular Weight:285.15
137-00-8

74-88-4

16311-69-6
General procedure: 4-methyl-5-hydroxyethyl thiazole (3.0 g, 21.0 mmol) was mixed with iodomethane (2.64 mL, 42.0 mmol) and heated to reflux for 2 hours. Upon completion of the reaction, excess iodomethane was removed by evaporation. Ether (50 mL) was added to the residual brown slurry and stirred for 30 minutes to promote crystallization. The precipitate was collected by filtration to afford 5-(2-hydroxyethyl)-3,4-dimethylthiazol-3-ium iodide (17) as a light yellow solid (5.76 g, 96.3% yield). The melting point of the product was 82-84 °C; 1H NMR (D2O, ppm): δ 4.14 (s, 3H, CH3N), 3.90 (t, 2H, CH2CH2O, J = 5.6 Hz), 3.19 (t, 2H, CH2CH2O, J = 6.0 Hz), 2.53 (s, 3H, CCH3).

137-00-8
531 suppliers
$8.00/10g

74-88-4
356 suppliers
$15.00/10g

16311-69-6
53 suppliers
$12.00/250mg
Yield:16311-69-6 96.3%
Reaction Conditions:
for 2 h;Reflux;
Steps:
5.1.12. N,4-Dimethyl-5-(2-(hydroxy) ethyl) thiazolium iodide (17)
5-(2-hydroxyethyl)-4-methylthiazole 7 (3.0 g, 21.0 mmol) and methyl iodide (2.64 mL, 42.0 mmol) were mixed and refluxed for 2 h. After evaporation of excess methyl iodide, to the residual brown syrup was added ether (50 mL) and stirred for 30 min, the precipitate was filtered to get 17 as a pale-yellow solid (5.76 g, 96.3%). Mp: 82-84 °C; 1H NMR (D2O, ppm): 4.14 (s, 3 H, CH3N), 3.90 (t, 2 H, CH2CH2O, J = 5.6 Hz), 3.19 (t, 2 H, CH2CH2O, J = 6.0 Hz), 2.53 (s, 3 H, CCH3).
References:
Fan, Wei;Wu, Yong;Li, Xian-Kun;Yao, Nian;Li, Xun;Yu, Yong-Guo;Hai, Li [European Journal of Medicinal Chemistry,2011,vol. 46,# 9,p. 3651 - 3661] Location in patent:experimental part