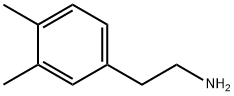
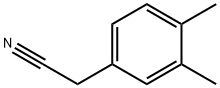
3,4-DIMETHYLPHENETHYLAMINE synthesis
- Product Name:3,4-DIMETHYLPHENETHYLAMINE
- CAS Number:17283-14-6
- Molecular formula:C10H15N
- Molecular Weight:149.23
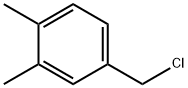
102-46-5

17283-14-6
Preparation Example 5: Synthesis of 2-(3,4-dimethylphenyl)ethylamine 1. 6.8 g of 90% sodium cyanide was dissolved in 6.5 mL of distilled water under heating conditions, followed by the addition of 15.5 g of 3,4-dimethylbenzyl chloride in 20 mL of anhydrous ethanol solution. 2. the resulting mixture was heated to boiling temperature and the reaction was maintained for 10 hours, after which it was cooled to room temperature. 3. The reaction solution was poured into 50 mL of distilled water and extracted twice with 150 mL of ether to combine the organic phases. 4. The organic solvent was removed by evaporation to give 11.5 g (79% yield) of 3,4-dimethylbenzyl cyanide. 5. the above product was dissolved in 50 mL of anhydrous tetrahydrofuran and slowly added to 6.0 g of LiAlH4 dispersion suspended in 150 mL of anhydrous tetrahydrofuran. 6. the mixture was heated to boiling point and reacted for 14 hours and then cooled to room temperature. 7. 16 mL of 1N NaOH and 8 mL of distilled water were slowly added to the reaction mixture. 8. The reaction mixture was filtered through a layer of diatomaceous earth, the solid was collected and dissolved in a mixture of 250 mL of ether and 250 mL of ethanol and filtered again. 9. The filtrate was alkalized with 5N NaOH aqueous solution and extracted with dichloromethane (200mL x 2). 10. The solvent was removed by distillation and purified by reduced pressure distillation to finally obtain 5.6 g (48% yield) of the target compound 2-(3,4-dimethylphenyl)ethylamine.

102-46-5
148 suppliers
$14.00/1g

17283-14-6
67 suppliers
$25.00/1g
Yield:17283-14-6 79%
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran;ethanol;water
Steps:
P.5 Preparation Example 5:
Preparation Example 5: Synthesis of 2-(3,4-dimethylphenyl)ethylamine To a solution of 6.8g of 90% sodium cyanide dissolved in 6.5ml of distilled water while being subjected to heating was added a solution of 15.5g of 3,4-dimethylbenzyl chloride in 20ml of dry ethanol; and the resultant mixture was heated at the boiling temperature for 10 hours and then cooled to room temperature. This solution was added to 50ml of distilled water and extracted twice with 150ml of ethyl ether. The organic solvent was removed to obtain 11.5g(yield 79%) of 3,4-dimethylbenzyl cyanide, which was dissolved in 50ml of dry tetrahydrofuran. The organic solution was slowly added to a dispersion of 6.0g of LiALH4 suspended in 150ml of dry tetrahydrofuran; and the mixture was heated at the boiling point for 14 hours. This reaction mixture was cooled to room temperature; and 16ml of 1N NaOH and 8ml of distilled water were slowly added thereto. Thereafter, this reaction mixture was filtered through a Celite layer to obtain solids, which were dissolved in a mixture of 250ml of ethyl ether and 250ml of ethyl alcohol and filtered. After the filtrate was basified with 5N NaOH aqueous solution and extracted with dichloromethane(200mlx2), the solvent was evaporated and distilled under reduced pressure to provide 5.6g(yield 48%) of the title compound.
References:
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY EP525360, 1993, A2
17283-17-9
0 suppliers
inquiry

17283-14-6
67 suppliers
$25.00/1g
17283-16-8
65 suppliers
$21.00/1g

17283-14-6
67 suppliers
$25.00/1g
3020-06-2
36 suppliers
inquiry

17283-14-6
67 suppliers
$25.00/1g
