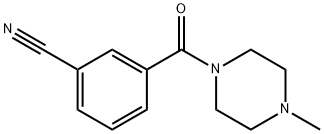
3-(4-Methylpiperazine-1-carbonyl)benzonitrile synthesis
- Product Name:3-(4-Methylpiperazine-1-carbonyl)benzonitrile
- CAS Number:1016743-09-1
- Molecular formula:C13H15N3O
- Molecular Weight:229.28
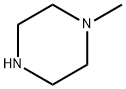
109-01-3
673 suppliers
$5.00/5G
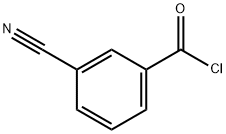
1711-11-1
112 suppliers
$17.00/250mg

1016743-09-1
9 suppliers
inquiry
Yield:1016743-09-1 99%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 6 h;
Steps:
106A.1 Step 1:
Step 1:
3-[(4-Methylpiperazin-1-yl)carbonyl]benzenecarbonitrile
A solution of 3-cyanobenzoic acid chloride in 100 ml of methylene chloride was added dropwise to a solution of 4.57 g (45.6 mmol) of 1-methylpiperazine and 8.5 ml (60.8 mmol) of triethylamine in 100 ml of methylene chloride at 0° C.
The mixture was then stirred at RT for 6 h.
200 ml of water were then added, the phases were separated and the organic phase was washed with water.
After drying over anhydrous magnesium sulfate, the mixture was filtered and the solvent was removed on a rotary evaporator. 6.9 g (99% of th.) of the title compound were obtained.
1H-NMR (400 MHz, CDCl3, δ/ppm): 7.72 (d, 1H), 7.70 (s, 1H), 7.65 (d, 1H), 7.55 (t, 1H), 3.80 (broad, 2H), 3.40 (broad, 2H), 2.50 (broad, 2H), 2.37 (broad, 2H), 2.34 (s, 3H).
LC/MS (method I, ESIpos): Rt=0.21 min, m/z=230 [M+H]+.
GC/MS (method L, ESIpos): Rt=7.36 min, m/z=229 [M]+.
References:
US2013/196964,2013,A1 Location in patent:Paragraph 1132; 1133; 1134; 1135; 1136