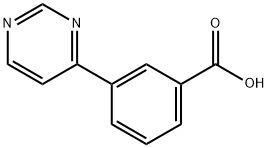
3-(5-Aminopyrimidin-4-yl)benzoic acid synthesis
- Product Name:3-(5-Aminopyrimidin-4-yl)benzoic acid
- CAS Number:856905-14-1
- Molecular formula:C11H8N2O2
- Molecular Weight:200.19
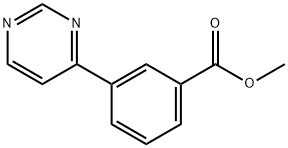
168619-01-0
17 suppliers
inquiry

856905-14-1
13 suppliers
$474.00/1g
Yield:856905-14-1 94%
Reaction Conditions:
Stage #1: 3-pyrimidin-4-yl-benzoic acid methyl esterwith sodium methylate in ethanol; for 0.5 h;Heating / reflux;
Stage #2: with hydrogenchloride in water; pH=~ 2;
Steps:
21
Methyl 3-bromobenzoate (110 g, 0.51 mol), dry acetonitrile (500 mL), ethynyl(trimethyl)silane (60.0 g, 0.61 mol), diisopropylamine (62.0 g, 0.61 mol) and tetra(triphenylphosphine)palladium (23.6 g, 0.02 mol) were placed under an atmosphere of argon in a three-necked round-bottomed 1 liter flask, equipped with a magnetic stirrer and a thermometer. The mixture was stirred for 30 minutes and then cooled to 10 0C. Copper iodide (9.7 g, 0.06 mol) was added, and the obtained suspension was stirred for a further 2.5 hours at 20 0C and finally for 3 hours at 60 0C. Then the mixture was left to stand at room temperature overnight and filtered. The precipitate of hydrobromide was washed with ether, and the combined filtrate was washed with saturated aqueous solutions of NH4CI and NaCI, dried over Na2SO4 and evaporated. The crude product was purified by chromatography (hexane) on a silica gel column to give methyl 3-(2- (trimethylsilyl)ethynyl)benzoate in 95% (112.8 g) yield.A suspension of methyl 3-(2-(trimethylsilyl)ethynyl)benzoate (112.8 g, 0.48 mol), mercury(2+) diacetate (16.2 g, 0.005 mol) in THF (350 mL) and concentrated H2SO4 (40 mL) was stirred at 60 0C for 3 hours. Then the mixture was cooled, diluted with ether (500 mL), filtered to remove precipitated mercury salts and washed to obtained neutral medium. Then the solution was dried over Na2SO4 and evaporated. The residue was purified by chromatography (hexane/ethyl acetate 4:1) on a silica gel column to give methyl 3-acetylbenzoate in 75% (65.2 g) yield.(Dimethoxymethyl)dimethylamine (90 mL) was added to a suspension of methyl 3-acetylbenzoate (65.2 g, 0.27 mol) in toluene (90 mL). The mixture was refluxed for 9 hours, during which time forming
References:
WO2008/65508,2008,A1 Location in patent:Page/Page column 37-38
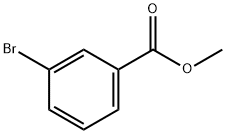
618-89-3
271 suppliers
$29.00/5g

856905-14-1
13 suppliers
$474.00/1g
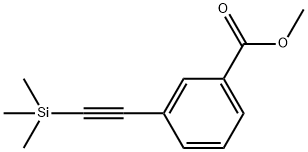
77123-59-2
16 suppliers
$351.00/1g

856905-14-1
13 suppliers
$474.00/1g
![Benzoic acid, 3-[3-(dimethylamino)-1-oxo-2-propen-1-yl]-, methyl ester](/CAS/20200611/GIF/168618-99-3.gif)
168618-99-3
2 suppliers
inquiry

856905-14-1
13 suppliers
$474.00/1g
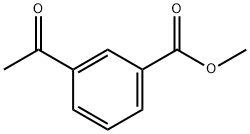
21860-07-1
80 suppliers
$15.00/250mg

856905-14-1
13 suppliers
$474.00/1g