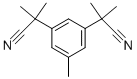
3,5-Bis(2-cyanoprop-2-yl)toluene synthesis
- Product Name:3,5-Bis(2-cyanoprop-2-yl)toluene
- CAS Number:120511-72-0
- Molecular formula:C15H18N2
- Molecular Weight:226.32
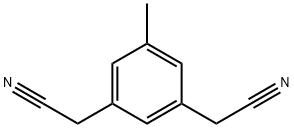
120511-74-2

74-88-4

120511-72-0
General procedure for the synthesis of 5,α,α,α,α',α'-pentamethyl-1,3-benzenediacetonitrile from 2,2'-(5-methyl-1,3-phenylene)bisacetonitrile and iodomethane: A mixture of 3,5-bis(cyano-methyl)toluene (800 g, 4.70 mol), methyl iodide (2,935.2 g, 20.68 mol) and dimethylformamide (11.20 L) was was cooled to 0°C to 5°C. A dispersion of 60% sodium hydride in mineral oil (864.2 g, 36.0 mol) was added in batches over 1 to 1.5 hours. Subsequently, the reaction mixture was gradually warmed to room temperature and stirred continuously for 2 to 2.5 hours. The progress of the reaction was monitored by thin layer chromatography (TLC, unfolding agent was hexane: ethyl acetate = 7.5:2.5). After confirming the completion of the reaction, ethyl acetate was added to quench the excess sodium hydride. The reaction mixture was diluted with water and extracted with dichloromethane (3 x 5 L). The organic phase was combined and washed with brine (5 L). The organic phase was decolorized with activated charcoal for 1 h at room temperature and subsequently concentrated to dryness under reduced pressure at 40°C to 45°C. The resulting residue was dissolved in carbon tetrachloride (2400 mL) at 70°C to 75°C and gradually cooled to 10°C to 15°C with stirring for 1 hour. The solution was further cooled to -5°C to 0°C and stirring was continued for 3 hours. Finally, the stirred suspension was filtered, the filter cake was washed with pre-cooled carbon tetrachloride (500 mL) and dried under vacuum at 40 °C to 45 °C to give 3,5-bis(1-cyano-1-methylethyl)toluene (748.0 g, 70.3% yield).

120511-74-2
177 suppliers
$39.00/25mg

74-88-4
357 suppliers
$15.00/10g

120511-72-0
279 suppliers
$11.00/250mg
Yield:120511-72-0 79%
Reaction Conditions:
with sodium hydride
Steps:
Preparation of 2,2 '-(5-methyl-1,3-phenylene)di(2-methylpropionitrile) 2Compound 2, 2,2'-(5-methyl-l,3-phenylene)di(2-methylpropionitrile), can be prepared according to the procedure in example 1 of patent EP 0296749 Bl, which is hereby incorporated by reference in its entirety, but in particular in respect of thepreparation of 2,2'-(5-methyl-l,3-phenylene)di(2-methylpropionitrile) 2. Yield: 79%. Melting point: 125-127°C.
References:
GENERICS [UK] LIMITED WO2006/836, 2006, A1 Location in patent:Page/Page column 2; 9-10; 14; Figure 2
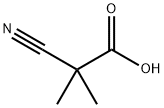
22426-30-8
79 suppliers
$89.70/250mg
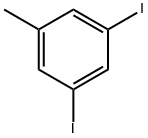
49617-79-0
22 suppliers
inquiry

120511-72-0
279 suppliers
$11.00/250mg

22426-30-8
79 suppliers
$89.70/250mg
1611-92-3
316 suppliers
$6.00/5g

120511-72-0
279 suppliers
$11.00/250mg

74-88-4
357 suppliers
$15.00/10g

120511-72-0
279 suppliers
$11.00/250mg

120511-74-2
177 suppliers
$39.00/25mg
80-48-8
459 suppliers
$5.00/25g

120511-72-0
279 suppliers
$11.00/250mg