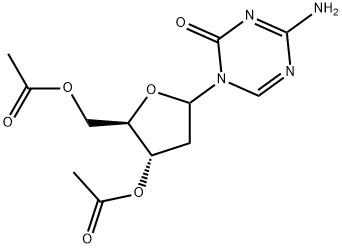
3',5'-di-o-acetyl-2-deoxy-5-azacytosine synthesis
- Product Name:3',5'-di-o-acetyl-2-deoxy-5-azacytosine
- CAS Number:22432-93-5
- Molecular formula:C12H16N4O6
- Molecular Weight:312.28
![N-(Trimethylsilyl)-4-[(trimethylsilyl)oxy]-2-amine-1,3,5-triazin](/CAS/GIF/52523-35-0.gif)
52523-35-0
34 suppliers
inquiry
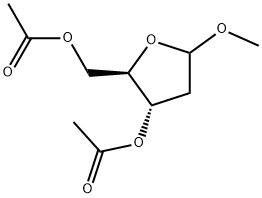
151767-35-0
33 suppliers
inquiry

1254186-96-3
3 suppliers
inquiry

22432-93-5
11 suppliers
inquiry
Yield:5.74 % de
Reaction Conditions:
Stage #1: 2-(trimethylsilylamino)-4-(trimethylsilyloxy)-s-triazine;3,5-di-O-acetyl-2-deoxy-α/β-D-ribofuranosidewith trimethylsilyl trifluoromethanesulfonate in dichloromethane at 0 - 30;
Stage #2: with sodium hydrogencarbonate in dichloromethane;Product distribution / selectivity;
Steps:
3
EXAMPLE 3: 1 -(3,5-di-O-acetyl-2-deoxy-D-ribofuranosyl)-5-azacytosine.5-Azacytosine (10 g) and acetonithle (50 mL) are charged into a round bottom flask at 25-350C and stirred for 5-10 minutes. Ammonium sulphate (0.49 g) and HMDS (38 mL) are added and the mixture is heated to 60-800C, maintained for 10-15 minutes, and stirred at 75-8O0C for 2 hours. The solvent is distilled completely under vacuum at 75-8O0C for 20-30 minutes, and dichloromethane (230 mL) and 1 -methoxy 3,5-di-O-acetyl-D-ribofuranose (20.71 g) are added to the residue and stirred for 5-10 minutes at 25-3O0C. The mass is cooled to 0-50C and TMS triflate (16.59 mL) is added and stirred for 10-15 minutes at 0-100C. The mass is gradually heated to 25-3O0C and stirred for 1 -1.5 hours. The mass is cooled to 10-200C and the reaction is quenched by adding 3% sodium carbonate solution (230 ml_), then the mixture is stirred for 5-10 minutes and layers are separated at 18-250C. The aqueous layer is extracted with dichloromethane (2χ230 ml_). The organic layers are combined and distilled at 35-450C for 35-40 minutes. Yield: 20.0 g (71.94%); Purity: 52.87% (β-anomer).
References:
WO2010/129211,2010,A2 Location in patent:Page/Page column 26-27