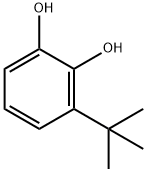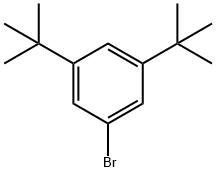
3,5-Di-tert-butylbromobenzene synthesis
- Product Name:3,5-Di-tert-butylbromobenzene
- CAS Number:22385-77-9
- Molecular formula:C14H21Br
- Molecular Weight:269.22
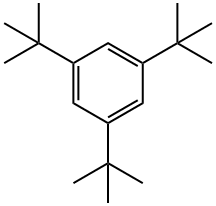
1460-02-2

22385-77-9
General procedure for the synthesis of 3,5-di-tert-butylbromobenzene from 1,3,5-tri-tert-butylbenzene: In a dry and argon-flushed 250 mL three-necked round-bottomed flask, a condenser (with nitrogen inlet connector), a thermometer, a dosing funnel, and a magnetic stirring bar coated with Teflon were assembled. Under nitrogen protection, 1,3,5-tri-tert-butylbenzene (15.0 g, 61 mmol, 1.0 eq.) was dissolved in 42 mL of dichloromethane, stirred, and cooled to 0°C. The solution was then purged with an argon flushing syringe. 1.0 mL (7.8 mmol, 0.13 equiv) of antimony pentachloride (SbCl5) was added slowly using an argon-flushed syringe. Liquid bromine (4.94 mL, 96.0 mmol, 1.6 equiv) was dissolved in 20 mL of dichloromethane and transferred to the addition funnel. The entire apparatus was wrapped in aluminum foil to protect it from light. The bromine solution was slowly added dropwise (approximately 1 drop every 2 seconds) over a period of 2 hours. After the dropwise addition, stirring was continued for 3 hours at 0°C. The reaction solution was poured into a mixture of 800 g of ice and 800 mL of water and stirred for 15 minutes. Adjust to pH 8-9 by slowly adding 100 mL of 5 M aqueous sodium hydroxide under stirring. extract the aqueous phase with dichloromethane three times using a 2 L partition funnel. The organic phases were combined and washed sequentially with water, saturated aqueous sodium thiosulfate, water and brine and dried over anhydrous magnesium sulfate. Concentrated by rotary evaporation and dried under vacuum for several hours. The crude product was recrystallized from 30 mL of hot ethanol, heated to boiling and then cooled to room temperature, the solid was collected by filtration and washed three times with minimal amounts of cold ethanol. Vacuum drying gave 11.1 g (68% yield) of 1-bromo-3,5-di-tert-butylbenzene.1H NMR (500 MHz, CDCl3): δ 1.30 (s, 18H), 7.17 (d, 1H), 7.32 (s, 2H). The analytical data are in agreement with literature reports.
Yield:-
Reaction Conditions:
with bromine in tetrachloromethane;
Steps:
Bromo-3,5-di-t-butylbenzene
Bromo-3,5-di-t-butylbenzene 1,3,5-Tri-t-butylbenzene (150 g, 0.6 mol) was dissolved in carbon tetrachloride (300 mL) in a three-necked flask which had been painted black to avoid light and equipped with an overhead stirrer, thermometer and addition funnel under argon. Iron pellets (36 g. 0.64 mol) were added and the slurry was cooled to 5°C. t-Butylcatechol (1.0 g) was added and a solution of bromine (201.6 g, 1.26 mol) in carbon tetrachloride (75 mL) was added over a one hour period. The slurry was stirred for an additional 4 hours at 5°C and quenched by pouring into ice water. The layers were separated and the organics washed with 10% sodium hydroxide solution. The solution then was washed with salt brine and dried over magnesium sulfate. The solvent was evaporated and the product was distilled under vacuum twice to give 75 g of product which was then recrystallized from heptane to give 47 g of pure product (29% yield).
References:
EP1157047,2003,B1

1460-02-2
223 suppliers
$6.00/5g

22385-77-9
301 suppliers
$5.00/1g
2380-36-1
143 suppliers
$57.39/1g

22385-77-9
301 suppliers
$5.00/1g
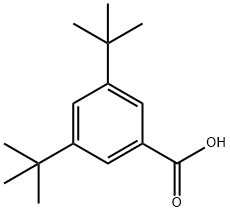
16225-26-6
152 suppliers
$7.00/250mg

22385-77-9
301 suppliers
$5.00/1g
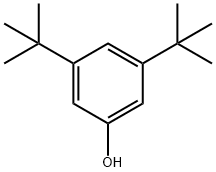
1138-52-9
159 suppliers
$8.00/100mg

22385-77-9
301 suppliers
$5.00/1g