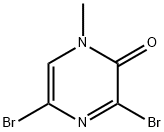
3,5-DibroMo-1-Methylpyrazin-2(1H)-one synthesis
- Product Name:3,5-DibroMo-1-Methylpyrazin-2(1H)-one
- CAS Number:87486-34-8
- Molecular formula:C5H4Br2N2O
- Molecular Weight:267.91
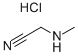
15219-34-8
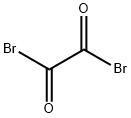
25808-30-4

87486-34-8
To a suspension of 2-methylaminoacetonitrile hydrochloride (20 g, 142.0 mmol) in dichloromethane (DCM, 1300 mL) was slowly added oxalyl bromide (54 mL, 188.67 mmol), followed by the dropwise addition of N,N-dimethylformamide (DMF, 1.5 mL) at 0 °C. The reaction mixture was warmed to 55 °C and stirred for 16 hours. Upon completion of the reaction, the dichloromethane was removed by distillation under reduced pressure and the crude product obtained was purified by column chromatography using 2% methanol/dichloromethane (MeOH/CH2Cl2) as eluent to afford the target compound 3,5-dibromo-1-methylpyrazin2(1H)-one as a light yellow solid (34 g, 92% yield). The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-d6): δ 8.1 (s, 1H), 3.4 (s, 3H).LC-MS analysis showed the molecular ion peak m/z 268.9 ([M + 1]+).

15219-34-8
78 suppliers
$100.00/25g

25808-30-4
166 suppliers
$6.00/5g

87486-34-8
69 suppliers
$19.00/250mg
Yield: 92%
Reaction Conditions:
in dichloromethane;N,N-dimethyl-formamide at 0 - 55; for 16 h;
Steps:
1 Synthesis of 3,5-dibromo-l-meth lpyrazin-2(lH)-one
To a suspension of methylamino acetonitrile hydrochloride (20 g, 142.0 mmol) in DCM (1300 ml), was added oxalyl bromide (54 ml, 188.67 mmol) followed by slow addition of DMF (1.5 ml) at 0°C. The reaction mixture was stirred at 55°C for 16 h. DCM was concentrated under reduced pressure, the residue obtained was purified by column chromatography by eluting with 2% MeOH/CH2Cl2 to afford the desired compound as a pale yellow solid (34 g, 92 %); *H NMR (400MHz, DMSO-dg) δ 8.1 (s, 1H), 3.4 (s, 3H). LC-MS: 268.9 (M+l)+.
References:
AURIGENE DISCOVERY TECHNOLOGIES LIMITED;BORUAH, Anima;HOSAHALLI, Subramanya;PANIGRAHI, Sunil Kumar WO2014/108820, 2014, A1 Location in patent:Page/Page column 27

15219-34-8
78 suppliers
$100.00/25g

5616-32-0
72 suppliers
$36.67/1g

87486-34-8
69 suppliers
$19.00/250mg

17509-60-3
0 suppliers
inquiry

5616-32-0
72 suppliers
$36.67/1g

87486-34-8
69 suppliers
$19.00/250mg

15219-34-8
78 suppliers
$100.00/25g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

87486-34-8
69 suppliers
$19.00/250mg

15219-34-8
78 suppliers
$100.00/25g

74-89-5
0 suppliers
$13.44/25ML

590-17-0
337 suppliers
$5.00/5g

87486-34-8
69 suppliers
$19.00/250mg