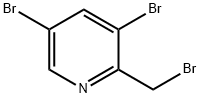
3,5-dibroMo-2-broMoMethyl-pyridine synthesis
- Product Name:3,5-dibroMo-2-broMoMethyl-pyridine
- CAS Number:1227502-56-8
- Molecular formula:C6H4Br3N
- Molecular Weight:329.81
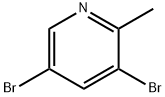
38749-87-0
118 suppliers
$15.00/250mg

1227502-56-8
5 suppliers
inquiry
Yield:1227502-56-8 55%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane; for 4 h;Heating / reflux;
Steps:
52B
To a solution of 52A (1.25 g, 4.98 mmol) in CCl4 (7 ML) was added NBS (0.89 g, 4.98 mmol) followed by catalytic AIBN and the mixture was heated to reflux for 4 hours. The reaction was cooled to room temperature, filtered and concentrated in vacuo. The residue was purified by flash chromatography (SiO2, 10% CH2Cl2/Hexane then 50% CH2Cl2/Hexane) to give 52B (0.90 g, 55%). HPLC Rt=2.85 min.
References:
US2005/192310,2005,A1 Location in patent:Page/Page column 35