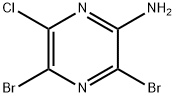
3,5-DIBROMO-6-CHLOROPYRAZIN-2-AMINE synthesis
- Product Name:3,5-DIBROMO-6-CHLOROPYRAZIN-2-AMINE
- CAS Number:566205-01-4
- Molecular formula:C4H2Br2ClN3
- Molecular Weight:287.34
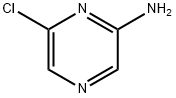
33332-28-4

566205-01-4
Synthesis Example 1 Preparation of 2-amino-3,5-dibromo-6-chloropyrazine (4): To a solution of 2-amino-6-chloropyrazine (3) (8.00 g, 61.8 mmol) in acetonitrile (80 mL) was slowly added N-bromosuccinimide (NBS) (27.5 g, 155 mmol) at 0 °C. After addition, the reaction mixture was gradually warmed to room temperature and stirred overnight (18 hours). After completion of the reaction, water was added to the mixture and the product was extracted with ether (3 x 80 mL). The organic phases were combined and washed sequentially with water (1 x 80 mL) and saturated saline (1 x 80 mL), then dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure and the resulting residue was purified by silica gel column chromatography (eluent: n-hexane/ethyl acetate= 3/1, v/v) to afford 2-amino-3,5-dibromo-6-chloropyrazine (4) (16.8 g, 58.5 mmol, 94.7% yield) as a yellow solid. tlc Rf = 0.31 (Expander: n-hexane/ethyl acetate= 4/1, v /v); 1H NMR (500MHz, CDCl3) δ 5.14 (s, 2H); 13C NMR (126MHz, CDCl3) δ 120.7, 122.0, 146.1, 151.0.

33332-28-4
299 suppliers
$9.00/1g

566205-01-4
86 suppliers
$19.00/250mg
Yield:566205-01-4 94.7%
Reaction Conditions:
with N-Bromosuccinimide in water;acetonitrile at 0 - 20; for 18 h;
Steps:
1
Synthesis Example 1 2-Amino-3,5-dibromo-6-chloropyrazine (4) To a solution of 2-amino-6-chloropyrazine (3) (8.00 g, 61.8 mmol) in acetonitrile (80 mL) was gradually added N-bromosuccinimide (NBS) (27.5 g, 155 mmol) at 0° C. After elevating to room temperature, the mixture was stirred overnight (18 hours). To the mixture was added water and the product was extracted with diethyl ether (*3). The combined organic extract was washed successively with water (*1) and brine (*1), followed by drying over anhydrous sodium sulfate. After filtration and concentration under reduced pressure, the residue was purified by silica gel flash column chromatography (n-hexane/ethyl acetate=3/1) to give 2-amino-3,5-dibromo-6-chloropyrazine (4) (16.8 g, 58.5 mmol, 94.7%) as a yellow solid. TLC Rf=0.31 (n-hexane/ethyl acetate=4/1); 1H NMR (500 MHz, CDCl3) δ 5.14 (s, 2H); 13C NMR (126 MHz, CDCl3) δ 120.7, 122.0, 146.1, 151.0.
References:
NATIONAL UNIVERSITY CORPORATION TOKYO MEDICAL AND DENTAL UNIVERSITY;JNC CORPORATION US2012/232272, 2012, A1 Location in patent:Page/Page column 18