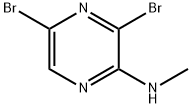
3,5-dibroMo-N-Methylpyrazin-2-aMine synthesis
- Product Name:3,5-dibroMo-N-Methylpyrazin-2-aMine
- CAS Number:894808-28-7
- Molecular formula:C5H5Br2N3
- Molecular Weight:266.92
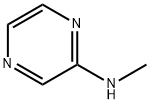
32111-28-7
50 suppliers
$24.00/250mg

894808-28-7
22 suppliers
$45.00/50mg
Yield:894808-28-7 44%
Reaction Conditions:
with N-Bromosuccinimide in water;dimethyl sulfoxide at 10 - 20;
Steps:
3; 25.B N-Methyl-3-(4-methyl-1,4-diazepan-1-yl)-5-(pyridin-4-yl)pyrazin-2-amine hydrochloride
To a stirred solution of aminopyrazine 4 (5.0 mmol) in dimethyl sulfoxide (10 mL)/water (0.20 mL) at 10° C. was added N-bromosuccinimide (10 mmol) in portions. The reaction mixture was then allowed to warm to room temperature slowly and stirred at that temperature overnight. An additional aliquot of N-bromosuccinimide (10 mmol) was then added at room temperature. After stirring for 6.5 h, the reaction mixture was poured onto ice (30 g). The precipitate was collected, washed with cold water (2×10 mL), and dried to provide the product 2, which could be purified by column chromatography, but was usually used in the next step without purification. ; Step B: 3,5-Dibromo-N-methylpyrazin-2-amine Prepared from the product of Step A according to general procedure 3 providing the dibromopyrazine (700 mg, 44%) as a yellow solid; 1H NMR (300 MHz, CDCl3) δ 8.07 (s, 1H), 5.26 (br s, 1H), 3.03-3.01 (d, J=5.0 Hz, 3H).
References:
US2006/142307,2006,A1 Location in patent:Page/Page column 5; 16
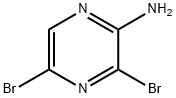
24241-18-7
442 suppliers
$6.00/5g

74-88-4
360 suppliers
$15.00/10g

894808-28-7
22 suppliers
$45.00/50mg