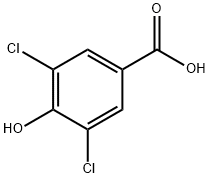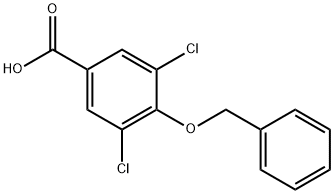
3,5-Dichloro-4-phenylmethoxybenzoic acid synthesis
- Product Name:3,5-Dichloro-4-phenylmethoxybenzoic acid
- CAS Number:41490-13-5
- Molecular formula:C14H10Cl2O3
- Molecular Weight:297.13

536974-83-1
5 suppliers
inquiry

41490-13-5
22 suppliers
inquiry
Yield:41490-13-5 97%
Reaction Conditions:
with potassium hydroxide in ethanol; for 48 h;Inert atmosphere;Reflux;
Steps:
4,4-bis(4-fluorophenyl)butyl 4-(benzyloxy)-3,5-dichlorobenzoate (36).
Potassium hydroxide (1.94 g, 34.70 mmol) was added to a solution of compound 35 (2.70 g, 8.68 mmol) in ethanol (50 mL), and reaction mixture was refluxed for 48 hours. After the reaction was completed, ethanol was evaporated under reduced pressure, and the obtained residue was resuspended in distilled water (50 mL). A clear aqueous solution obtained upon filtration was acidified with 1N HCl (pH 3) and filtered again to obtain the desired product 36 (2.50 g, 97%) as white solid. 1H NMR (400 MHz, CDCl3) δH 5.10 (s, 2H), 7.42 (d, 3H, J = 7.58 Hz), 7.53 (d, 2H, J = 6.72 Hz), 7.95 (s, 2H), 13.58 (br. s., 1H).
References:
Ashraf-Uz-Zaman, Md;Shahi, Sadisna;Akwii, Racheal;Sajib, Md Sanaullah;Farshbaf, Mohammad Jodeiri;Kallem, Raja Reddy;Putnam, William;Wang, Wei;Zhang, Ruiwen;Alvina, Karina;Trippier, Paul C.;Mikelis, Constantinos M.;German, Nadezhda A. [European Journal of Medicinal Chemistry,2021,vol. 209,art. no. 112866] Location in patent:supporting information

40689-39-2
4 suppliers
inquiry

41490-13-5
22 suppliers
inquiry

100-39-0
429 suppliers
$10.00/10g

41490-13-5
22 suppliers
inquiry
3337-59-5
110 suppliers
$6.00/250mg

41490-13-5
22 suppliers
inquiry