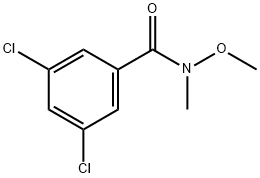
3,5-dichloro-N-Methoxy-N-MethylbenzaMide synthesis
- Product Name:3,5-dichloro-N-Methoxy-N-MethylbenzaMide
- CAS Number:259796-12-8
- Molecular formula:C9H9Cl2NO2
- Molecular Weight:234.08
2905-62-6
369 suppliers
$13.43/1gm:

6638-79-5
589 suppliers
$6.00/25g

259796-12-8
37 suppliers
inquiry
Yield:259796-12-8 95%
Reaction Conditions:
with pyridine in dichloromethane at 0; for 5 h;
Steps:
4.A
3,5-Dichlorobenzoyl chloride (10.0 g, 47.7 mmoles) and N,O-dimethylhyoxylamine hydrochloride (4.23 g, 43.4 mmoles) were dissolved in anhydrous dichloromethane (475 mL) and the mixture was cooled to 0° C. under argon. Anhydrous pyridine (7.55 g, 7.79 mL, 95.5 mmoles) was added dropwise to the stirred solution at 0° C. and the mixture was stirred at 0° C. for 5 h. The mixture was evaporated to dryness and the residue was partitioned between diethyl ether-dichloromethane (1:1) and brine. The organic layer was dried (MgSO4), filtered and evaporated to dryness. The residue was chromatographed on a silica gel column (60×5 cm) using 0.75% (10% conc. NH4OH in methanol)-dichloromethane as the eluant to give 3,5-dichloro-N-methoxy-N-methylbenzamide as a colorless oil (9.66 g, 95%): FABMS: m/z 234.2 (MH+); HRFABMS: m/z 234.0090 (MH+), Calcd. for C9H10Cl2NO2: m/z 234.0089; 8H (CDCl3) 3.34 (3H, s, NCH3), 3.54 (3H, s, OCH3), 7.44 (1H, dd, H4), 7.56 ppm (2H, d, H2 and H6); δC (CDCl3) CH3: 33.5, 61.5; CH: 126.9, 126.9, 130.6; C, 134.8, 134.8, 136.7, 166.8.
References:
US2007/15774,2007,A1 Location in patent:Page/Page column 208
51-36-5
387 suppliers
$7.00/25g

259796-12-8
37 suppliers
inquiry