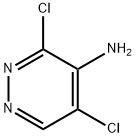
3,5-dichloropyridazin-4-amine synthesis
- Product Name:3,5-dichloropyridazin-4-amine
- CAS Number:53180-76-0
- Molecular formula:C4H3Cl2N3
- Molecular Weight:163.99
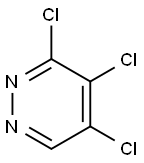
14161-11-6

53180-76-0
General procedure for the synthesis of 4-amino-3,5-dichloropyridazine from 3,4,5-trichloropyridazine: 3,4,5-trichloropyridazine (3,500 mg, 2.73 mmol) was dissolved in a mixture of ethanol (5.5 mL) and ammonium hydroxide (5.5 mL) in a microwave reactor and heated at 120 °C for 25 min. After completion of the reaction, the reaction solution was concentrated under reduced pressure to remove the solvent. The crude product was purified by silica gel column chromatography, and the eluent was a mixed solution of acetone and dichloromethane (acetone volume fraction 0-15%). 4-Amino-3,5-dichloropyridazine was finally obtained in 36% yield (163 mg). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 5.11 (broad peak, 2H), 8.74 (single peak, 1H).LC-MS analysis showed a retention time of Rt = 0.27 min and a mass spectrum m/z 164 [M + H]+.

14161-11-6
151 suppliers
$9.00/1g

53180-76-0
84 suppliers
$61.00/100mg
Yield:53180-76-0 36%
Reaction Conditions:
with ammonium hydroxide in ethanol at 120; for 0.416667 h;Microwave irradiation;
Steps:
4 3,5-Dichloropyridazin-4-amine
A mixture of 3,4,5-trichloropyridazine (Preparation 3, 500 mg, 2.73 mmole) in EtOH (5.5 mL) and NH4OH (5.5 mL) was heated under microwave irradiation 120° C. for 25 minutes. Concentration under reduced pressure and purification via silica gel column chromatography eluting with acetone:dichloromethane (0-15% acetone), provided the title product in 36% yield, 163 mg. 1H NMR (400 MHz, CDCl3): δ ppm 5.11 (br s, 2H), 8.74 (s, 1H). LCMS Rt=0.27 minutes MS m/z 164 [M+H]+
References:
US2014/171435,2014,A1 Location in patent:Paragraph 0454; 0455; 0456

14161-11-6
151 suppliers
$9.00/1g
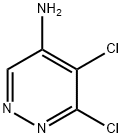
89180-50-7
63 suppliers
$39.00/100mg

53180-76-0
84 suppliers
$61.00/100mg

14161-11-6
151 suppliers
$9.00/1g

7664-41-7
522 suppliers
$15.00/100ml

89180-50-7
63 suppliers
$39.00/100mg

53180-76-0
84 suppliers
$61.00/100mg