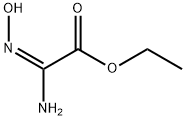
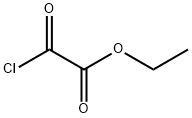
3,5-Diethyl 1,2,4-oxadiazole-3,5-dicarboxylate synthesis
- Product Name:3,5-Diethyl 1,2,4-oxadiazole-3,5-dicarboxylate
- CAS Number:40019-27-0
- Molecular formula:C8H10N2O5
- Molecular Weight:214.18
Yield:40019-27-0 99%
Reaction Conditions:
with pyridine in chloroform at 0 - 20; for 4.5 h;Reflux;
Steps:
Diethyl 1,2,4-oxadiazole-3,5-dicarboxylate (33).69
A 1-l,three-neck flask equipped with a magnetic stir bar, refluxcondenser, drying tube, dropping funnel, and thermometerwas charged with ethyl 2-amino-2-(hydroxyimino)acetate(32)74 (32.0 g, 242 mmol) and CHCl3 (500 ml). Anhydrouspyridine (59.0 ml, 732 mmol) was added to the slurry, andthe reaction mixture was cooled with an ice bath (internaltemperature 5-10°C). Ethyl chlorooxoacetate (40.6 ml,363 mmol) was added over a period of 1 h from thedropping funnel at such a rate to maintain the reactiontemperature around 10°C. The cooling bath was removed,the reaction mixture was stirred at room temperature for30 min and then refluxed for 3 h. A fine precipitateimpurity was formed during reflux, which was filtered offafter cooling to room temperature. The CHCl3 solution wasdiluted with CH2Cl2 and washed successively with 1 N HCl(3×300 ml), H2O (2×300 ml), and brine (2×300 ml). Theorganic layer was dried with anhydrous Na2SO4, and theorganic solution concentrated to give product 33 that wasused in the next step without any further purification. Yield52 g (>99%), colorless oil. IR spectrum (ATR, neat), ν, cm-1:1752 (C=O). 1H NMR spectrum (600 MHz, CDCl3),δ, ppm: 4.67-4.41 (4H, m, 2CH2); 1.54-1.37 (6H, m,2CH3). 13C NMR spectrum (150 MHz, CDCl3), δ, ppm:167.7; 162.6; 156.5; 153.2; 64.3; 63.4; 14.0; 13.9.
References:
Pagoria, Philip F.;Zhang, Mao-Xi;Zuckerman, Nathaniel B.;DeHope, Alan J.;Parrish, Damon A. [Chemistry of Heterocyclic Compounds,2017,vol. 53,# 6-7,p. 760 - 778][Khim. Geterotsikl. Soedin.,2017,vol. 53,p. 760 - 778,19]