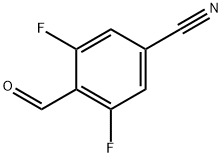
3,5-DIFLUORO-4-FORMYLBENZONITRILE synthesis
- Product Name:3,5-DIFLUORO-4-FORMYLBENZONITRILE
- CAS Number:467442-15-5
- Molecular formula:C8H3F2NO
- Molecular Weight:167.11
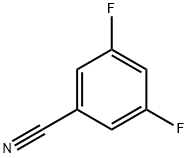
64248-63-1
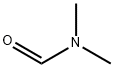
68-12-2

467442-15-5
Under nitrogen protection, 2.5 M tert-butyllithium (4.4 mL) was slowly added dropwise to a stirred solution of N,N-diisopropylethylamine (1.1 g, 11 mmol) in anhydrous tetrahydrofuran (100 mL), keeping the reaction temperature at -78 °C. The reaction system was then slowly warmed to 0 °C and stirred continuously for 1 hour. After that, the reaction solution was re-cooled to -78 °C and 3,5-difluorobenzonitrile (1.39 g, 10 mmol) was added. After continued stirring at -78 °C for 1 h, N,N-dimethylformamide (877 mg, 12 mmol) was added to the reaction solution and the reaction was carried out for 0.5 h. The reaction was carried out at -78 °C for 0.5 h. Upon completion of the reaction, the reaction was quenched by the addition of 10% v/v aqueous acetic acid (20 mL). The reaction mixture was gradually warmed up to room temperature, extracted with ethyl acetate, and concentrated under reduced pressure after combining the organic phases to afford 3,5-difluoro-4-formylbenzonitrile (1.29 g, 77% yield) as a yellow solid.
![Benzonitrile, 3,5-difluoro-4-[(methylsulfinyl)(methylthio)methyl]-](/CAS/20210305/GIF/433939-87-8.gif)
433939-87-8
0 suppliers
inquiry

467442-15-5
107 suppliers
$18.00/50mg
Yield: 98%
Reaction Conditions:
with sulfuric acid in tetrahydrofuran at 20; for 72 h;
Steps:
B.(ii) (ii) 2,6-Difluoro-4-formylbenzonitrile
2,6-Difluoro-4 [ (methylsulfinyl) (methylthio) methyl] benzonitrile (2.17 g, 8.32 mmol; see step (i) above) was dissolved in 90 mL of THF and 3.5 mL of concentrated sulfuric acid was added. The mixture was left at room temperature for 3 days and subsequently poured into 450 mL of water. Extraction three times with EtOAc followed and the combined ethereal phase was washed twice with aqueous sodium bicarbonate and with brine, dried [(NA2SO4)] and evaporated. Yield: 1.36 g [(98%).] The position of the formyl group was established by 13C NMR. The signal from the fluorinated carbons at 162.7 ppm exhibited the expected coupling pattern with two coupling constants in the order of 260 Hz and 6.3 Hz respectively corresponding to an ipso and a meta coupling from the fluorine atoms. 'H NMR (400 MHz, [CDC13)] [8] 10.35 (s, 1H), 7.33 (m, 2H)
References:
ASTRAZENECA AB WO2003/101423, 2003, A1 Location in patent:Page 25