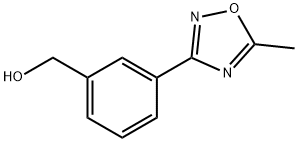
[3-(5-METHYL-1,2,4-OXADIAZOL-3-YL)PHENYL]METHANOL synthesis
- Product Name:[3-(5-METHYL-1,2,4-OXADIAZOL-3-YL)PHENYL]METHANOL
- CAS Number:852180-70-2
- Molecular formula:C10H10N2O2
- Molecular Weight:190.2
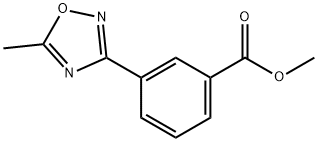
1092566-65-8
27 suppliers
$441.00/5g
![[3-(5-METHYL-1,2,4-OXADIAZOL-3-YL)PHENYL]METHANOL](/CAS/GIF/852180-70-2.gif)
852180-70-2
24 suppliers
$172.22/250mg
Yield:852180-70-2 54%
Reaction Conditions:
Stage #1: methyl 3-(5-methyl-1,2,4-oxadiazol-3-yl)benzoatewith lithium borohydride in tetrahydrofuran; for 20 h;Cooling with ice;
Stage #2: with hydrogenchloride in tetrahydrofuran;water; pH=7;
Steps:
6.b
b)3.46 g of methyl 3-(5-methyl-1,2,4-oxadiazol-3-yl)benzoate (15.86 mmol) are dissolved in absolute THF in a 250 ml three-necked flask, 0.691 g of LiBH4 (31.71 mmol) are introduced in portions with ice/H2O cooling and stirring, and the mixture is stirred without cooling for a further 20 h. Work-up: the pH is adjusted to 7 by slow dropwise addition of 1 N HCl (vigorous foaming) with stirring, the mixture is diluted with 100 ml of H2O and shaken with 3×50 ml of dichloromethane, the combined extracts are washed with 100 ml of H2O, dried over sodium sulfate and evaporated to dryness, and the residue is purified by chromatography.The crude chromatography residue is recrystallised from diethyl ether/petroleum ether.Yield: 1.643 g (8.64 mmol)=54% of [3-(5-methyl-1,2,4-oxadiazol-3-yl)-phenyl]methanol; m.p. 57-58°; ESI 191 (M+H); HPLC: Rt=2.88 min (method C).
References:
US2010/280030,2010,A1 Location in patent:Page/Page column 34
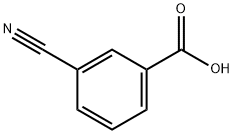
1877-72-1
281 suppliers
$7.00/5g
![[3-(5-METHYL-1,2,4-OXADIAZOL-3-YL)PHENYL]METHANOL](/CAS/GIF/852180-70-2.gif)
852180-70-2
24 suppliers
$172.22/250mg
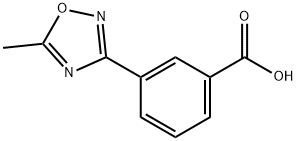
264264-32-6
91 suppliers
$45.00/50mg
![[3-(5-METHYL-1,2,4-OXADIAZOL-3-YL)PHENYL]METHANOL](/CAS/GIF/852180-70-2.gif)
852180-70-2
24 suppliers
$172.22/250mg
![3-[(HYDROXYAMINO)IMINOMETHYL]-BENZOIC ACID](/CAS/GIF/199447-10-4.gif)
199447-10-4
60 suppliers
$45.00/250mg
![[3-(5-METHYL-1,2,4-OXADIAZOL-3-YL)PHENYL]METHANOL](/CAS/GIF/852180-70-2.gif)
852180-70-2
24 suppliers
$172.22/250mg