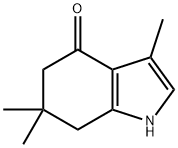
3,6,6-trimethyl-6,7-dihydro-1H-indol-4(5H)-one synthesis
- Product Name:3,6,6-trimethyl-6,7-dihydro-1H-indol-4(5H)-one
- CAS Number:56008-20-9
- Molecular formula:C11H15NO
- Molecular Weight:177.24
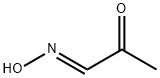
17280-41-0
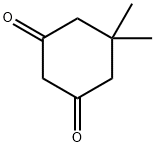
126-81-8

56008-20-9
Step 1. Synthesis of 3,6,6-trimethyl-6,7-dihydro-1H-indol-4(5H)-one: (E)-acetone aldehyde-1-oxime (0.50 g, 5.74 mmol) and 5,5-dimethyl-1,3-cyclohexanedione (0.80 g, 5.74 mmol) were dissolved in a mixed solvent of acetic acid (35 mL) and water (15 mL). Zinc powder (0.75 g, 11.5 mmol) was slowly added to the reaction system at room temperature. The reaction mixture was refluxed under stirring conditions. After completion of the reaction, the reaction solution was concentrated under reduced pressure and subsequently extracted with dichloromethane and saturated brine. The pH of the aqueous phase was adjusted with saturated sodium bicarbonate solution to 6. The aqueous phase was again extracted with dichloromethane and the organic layers were combined and dried over anhydrous magnesium sulfate. After filtration to remove the desiccant, the filtrate was concentrated under reduced pressure to give the crude product. Purification of the crude product by silica gel column chromatography (eluent: hexane/ethyl acetate, 1/3) afforded 3,6,6-trimethyl-6,7-dihydro-1H-indol-4(5H)-one as a yellow solid (4.9 g, 52% yield).

17280-41-0
12 suppliers
inquiry

126-81-8
424 suppliers
$5.00/10g

56008-20-9
46 suppliers
$41.00/100mg
Yield:56008-20-9 52%
Reaction Conditions:
with acetic acid;zinc in water;Reflux;
Steps:
45.1 Step 1. Synthesis of 3,6,6-trimethyl-6,7-dihydro-lH-indole-4(5H)-on [formula 6-2]
Step 1. Synthesis of 3,6,6-trimethyl-6,7-dihydro-lH-indole-4(5H)-on [formula 6-2]Anti-pyruvic aldehyde- 1-oxime [formula 6-1] (0.50 g, 5.74 mmol) and 5,5-dimethyl- 1,3-cyclohexandion [formula 6-7] (0.80 g, 5.74 mmol) were dissolved in acetic acid (35 mL) and H20 (15 mL) . Thereto, zinc powder (0.75 g, 11.5 mmol) was added slowly maintaining room temperature. The reaction mixture was refluxed with stirring, concentrated under reduced pressure and extracted with CH2C12 and brine, of which pH was adjusted to 6 using saturated NaHC03. The reaction mixture was extracted with CH2C12; organic layer was dried over anhydrous MgS04 and filtered. Residue was concentrated under reduced pressure and purified by column chromatography (Si02; hexane/ethylacetate, 1/3) to yield the title compound as yellow solid (4.9 g, 52 %).
References:
WO2013/62344,2013,A1 Location in patent:Page/Page column 87

126-81-8
424 suppliers
$5.00/10g
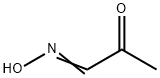
306-44-5
59 suppliers
$15.00/250mg

56008-20-9
46 suppliers
$41.00/100mg
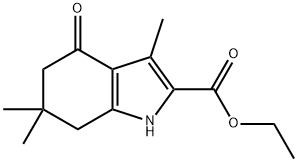
37711-24-3
23 suppliers
$175.00/1g

56008-20-9
46 suppliers
$41.00/100mg

126-81-8
424 suppliers
$5.00/10g

56008-20-9
46 suppliers
$41.00/100mg
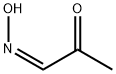
31915-82-9
84 suppliers
$27.00/1g

126-81-8
424 suppliers
$5.00/10g

56008-20-9
46 suppliers
$41.00/100mg