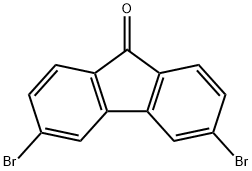
3,6-Dibromo-fluoren-9-one synthesis
- Product Name:3,6-Dibromo-fluoren-9-one
- CAS Number:216312-73-1
- Molecular formula:C13H6Br2O
- Molecular Weight:337.99
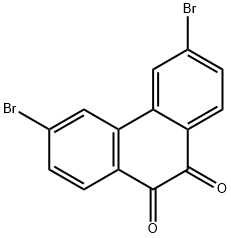
53348-05-3

216312-73-1
General procedure for the synthesis of 3,6-dibromo-9,10-phenanthrenequinone from 3,6-dibromo-9H-fluoren-9-one: 60.0 g (1.0 mol) of potassium hydroxide was dissolved in 400 ml of water, followed by the addition of 13.0 g (35.0 mmol) of 3,6-dibromoquinolinone obtained from step 1 to a 1000 ml three-necked flask. The reaction mixture was heated to 100 °C and kept at this temperature for 3 hours. During the reaction, 30.0 g (200.0 mmol) of potassium permanganate was added in batches and the reaction was continued for 10 hours after addition. Upon completion of the reaction, the mixture was cooled to room temperature. Subsequently, solid sodium thiosulfate powder was added to the reaction solution and the pH was adjusted to neutral, at which point a black solid precipitated. The precipitate was collected by filtration and the filter cake was wrapped in filter paper and placed in a Soxhlet extractor and extracted with dichloromethane for 3 consecutive days. After the extraction was completed, the dichloromethane solution was rotary evaporated to dryness to give 4.8 g of a light yellow solid, i.e., the intermediate 3,6-dibromo-9H-fluoren-9-one, in 40% yield.

53348-05-3
142 suppliers
$6.00/250mg

216312-73-1
75 suppliers
$9.00/250mg
Yield:216312-73-1 88%
Reaction Conditions:
with potassium permanganate;potassium hydroxide in water at 130; for 1 h;Inert atmosphere;
Steps:
3,6-Dibromo-9-fluorenone
KOH (4.0 g, 71.3 mol) was dissolved in water (15 mL) and the mixture was heated to 130 °C. Then, 3,6-dibromophenanthrene-9,10-dione (2.0 g, 5.4 mmol) was added to the solution. When the mixture is completely black, KMnO4 (4.2 g, 26.6 mmol) was added slowly and carefully. After addition, the reaction mixture was stirred at 130 °C for 1 h and cooled to room temperature. Sodium thiosulfate was added carefully to the mixture until the purple fades. Then, concentrated sulfuric acid was added drop by drop to the mixture until it turned light yellow. The precipitate was filtered, washed with water and dried under vacuum. The target product was obtained as a light yellow power (1.6 g, 88%). 1H NMR (400 MHz, CDCl3): δ (ppm) 7.67 (d, J = 1.6 Hz, 2H), 7.55 (d, J = 7.6 Hz, 2H), 7.50 (dd, J = 8.0, 1.6 Hz, 2H).
References:
Tian, Zhuanzhuan;Yang, Xiaolong;Liu, Boao;Zhong, Daokun;Zhou, Guijiang [Journal of Organometallic Chemistry,2019,vol. 895,p. 28 - 36] Location in patent:supporting information
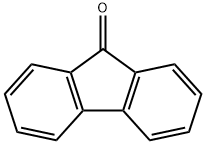
486-25-9
488 suppliers
$5.00/25g

216312-73-1
75 suppliers
$9.00/250mg
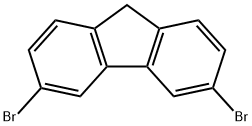
500901-89-3
28 suppliers
inquiry

216312-73-1
75 suppliers
$9.00/250mg
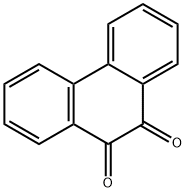
84-11-7
253 suppliers
$5.00/1g

216312-73-1
75 suppliers
$9.00/250mg

83740-04-9
5 suppliers
inquiry

216312-73-1
75 suppliers
$9.00/250mg