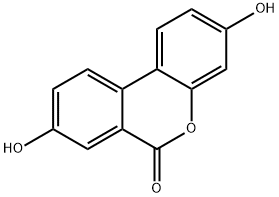
Urolithin A synthesis
- Product Name:Urolithin A
- CAS Number:1143-70-0
- Molecular formula:C13H8O4
- Molecular Weight:228.2
the intermediate product, "Intermediate A", is treated with the Lewis acid, AlCl3, in order to obtain a crude urolithin A. The ether cleavage of Intermediate A is accomplished by activation of the methyl ether through the addition of the Lewis acid, AlCl3, in toluene. The activated species is then hydrolyzed by the addition of water. Following hydrolysis, the crude product is filtered and dried.
crude urolithin A is dissolved in dimethyl sulfoxide (DMSO) for a polish filtration and subsequently precipitated from this DMSO solution by the addition of water. The filter cake is rinsed by water, followed by methanol. The raw urolithin A is then triturated with acetic acid (HOAc) to further purify the product and then collected by filtration. Following filtration, the purified product is rinsed with HOAc followed by tert-butyl-methyl ether (TBME), then dried to yield the final product, urolithin A.
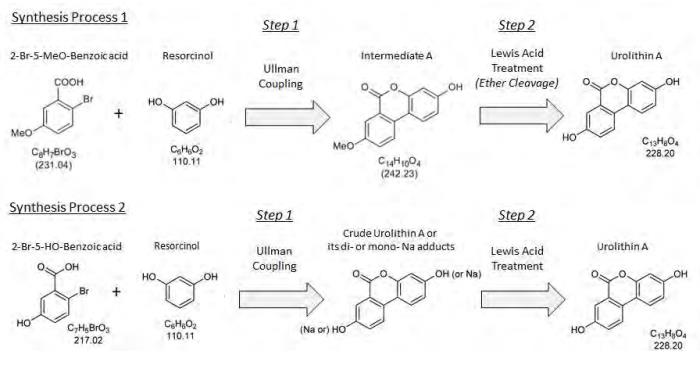
Synthesis of Urolithin A
![3,8-dimethoxybenzo[c]chromen-6-one](/CAS/20180719/GIF/1680-85-9.gif)
1680-85-9
3 suppliers
inquiry

1143-70-0
364 suppliers
$30.00/1mg
Yield:1143-70-0 64%
Reaction Conditions:
with boron tribromide in dichloromethane at 0;Inert atmosphere;
Steps:
1 4.7 General procedure for the preparation of urolithins A-C (2a-c)
General procedure: A solution of methyl ether of urolithins A-C 30a-c (1.0equiv) was cooled at 0°C under Ar atmosphere and was added BBr3 slowly. Once the stating material was completely consumed by TLC, a solution of 2N HCl was added to acidify and partitioned with EtOAc to give the crude product. The crude product was purified by size exclusion chromatography to obtain the product.
References:
Nealmongkol, Prattya;Tangdenpaisal, Kassrin;Sitthimonchai, Somkid;Ruchirawat, Somsak;Thasana, Nopporn [Tetrahedron,2013,vol. 69,# 44,p. 9277 - 9283]
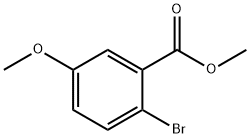
35450-36-3
188 suppliers
$10.00/1g
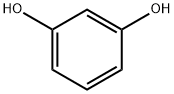
108-46-3
793 suppliers
$10.00/10g

1143-70-0
364 suppliers
$30.00/1mg
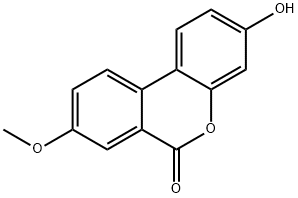
35233-17-1
39 suppliers
$120.00/0.500g

1143-70-0
364 suppliers
$30.00/1mg
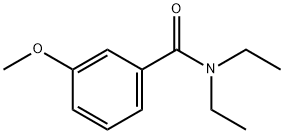
62924-93-0
5 suppliers
inquiry

1143-70-0
364 suppliers
$30.00/1mg

133730-33-3
2 suppliers
inquiry

1143-70-0
364 suppliers
$30.00/1mg