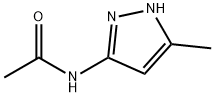
3-Acetamido-5-methylpyrazole synthesis
- Product Name:3-Acetamido-5-methylpyrazole
- CAS Number:83725-05-7
- Molecular formula:C6H9N3O
- Molecular Weight:139.16
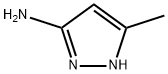
31230-17-8

108-24-7

83725-05-7
4.1.1 Synthesis of N-(5-methyl-1H-pyrazol-3-yl)-acetamide (2): 5-methyl-1H-pyrazol-3-amine (1) (10 g, 103 mmol) was dissolved in 100 mL of distilled water and sodium bicarbonate (NaHCO3) (26 g, 309 mmol) was added slowly. Subsequently, acetic anhydride (19.5 mL, 206 mmol) was added dropwise and the resulting suspension was heated and refluxed for 16 hours (the reaction process was monitored by thin layer chromatography (TLC)). Upon completion of the reaction, the mixture was cooled to room temperature and white crystals were observed to precipitate slowly. The precipitate was collected by filtration, washed with distilled water and dried to afford the target product N-(5-methyl-1H-pyrazol-3-yl)-acetamide (2) (6.2 g, 44 mmol, 43% yield), which did not require further purification. The melting point of the product was 210-211 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.92 (s, 1H), 10.19 (s, 1H), 6.24 (s, 1H), 2.17 (s, 3H), 1.96 (s, 3H).

31230-17-8
364 suppliers
$5.00/5g

108-24-7
0 suppliers
$14.00/250ML

83725-05-7
49 suppliers
$24.00/100mg
Yield:83725-05-7 62%
Reaction Conditions:
Stage #1: 3-methyl-5-aminopyrazolewith sodium hydrogencarbonate in water at 20; for 0.5 h;
Stage #2: acetic anhydride in water at 100; for 3 h;
Steps:
1.1 Step 1: Synthesis of N-(5-methyl-1H-pyrazol-3-yl)acetamide
5-methyl-1H-pyrazol-3-amine (300 g, 3.09 mol, 1.0 eq) was weighted into a 5 L round bottom flask, dissolved by adding water (2800 mL) with mechanical stirring at room temperature. Sodium bicarbonate (780 g, 9.28 mol, 3 eq) was added in portions, following by stirring for another 30 min after the addition. Then, acetic anhydride (592 ml, 6.2 mol, 2 eq) was added dropwise and slowly to the reaction system over about 1 h at controlled drop rate. At this point, a large amount of foamy white solid was produced. The temperature was raised to 100° C. and the reaction system was stirred for 2 h. The solid was gradually dissolved, and the system became clarification. Then heating was stopped, and the system was cooled to room temperature. After stirring overnight, a large amount of white crystalline solid precipitated. Another batch of 5-methyl-1H-pyrazol-3-amine (300 g) was fed in a parallel reaction. After the reactions were completed, the two batches were combined and filtered, and the solid was washed with water (500 mL*2) and dried to give a white solid (554 g, yield 62%). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 10.32 (s, 1H), 6.21 (s, 1H), 2.18 (s, 3H), 1.97 (s, 3H).
References:
US2019/40064,2019,A1 Location in patent:Paragraph 0155; 0156

31230-17-8
364 suppliers
$5.00/5g

83725-05-7
49 suppliers
$24.00/100mg

31230-17-8
364 suppliers
$5.00/5g

75-36-5
589 suppliers
$17.92/100G

83725-05-7
49 suppliers
$24.00/100mg