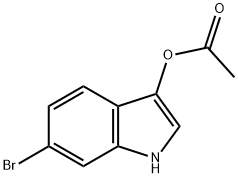
3-acetoxy-6-broMoindole synthesis
- Product Name:3-acetoxy-6-broMoindole
- CAS Number:114306-17-1
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08

64-19-7
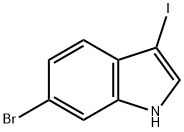
372077-73-1

114306-17-1
General procedure for the synthesis of 6-bromo-1H-indol-3-yl acetate from glacial acetic acid and compound (CAS:372077-73-1): silver acetate (341 mg, 2.04 mmol) was added to a solution of glacial acetic acid (8 mL) of monomer 2. After stirring the reaction mixture at 90°C for 3 h, it was cooled to room temperature and filtered. The filtrate was concentrated by evaporation under reduced pressure. The residue was purified by silica gel column chromatography using dichloromethane as eluent to afford the target product 6-bromo-1H-indol-3-yl acetate (yield: 254 mg, yield: 63.0%). The structure of the product was confirmed by 1H NMR (CDCl3, 600 MHz) and 13C NMR (CDCl3, 600 MHz) as follows: 1H NMR (CDCl3, 600 MHz): δ 7.97 (s, 1H), 7.69 (d, 1H), 7.33 (d, 1H), 7.29 (m, 1H), 7.17 (m, 1H) , 2.36 (s, 9H); 13C NMR (CDCl3, 600 MHz): δ 168.65, 131.70, 129.82, 125.78, 121.61, 120.14, 114.64, 113.17, 112.90, 20.93.

64-19-7
1628 suppliers
$10.00/25ML

372077-73-1
15 suppliers
inquiry

114306-17-1
41 suppliers
$125.00/100mg
Yield: 254 mg
Reaction Conditions:
with silver(I) acetate at 90; for 3 h;
Steps:
3-Acetoxy-6-bromoindole (3)
Silver acetate (341 mg,2.04 mmol) was added to a solution of monomer 2 in acetic acid(8 mL). After stirring for 3 h at 90 C, the mixture was cooled toroom temperature and filtered. The filtrate was evaporated todryness under reduced pressure. The residue was chromatographedon silica gel with dichloromethane as eluent to affordproduct 3 (yield: 254 mg, 63.0%). 1H NMR (CDCl3, 600 MHz): 7.97 (s,1H), 7.69 (d, 1H), 7.33 (d, 1H), 7.29 (m, 1H), 7.17 (m, 1H), 2.36 (s, 9H);13C NMR (CDCl3, 600 MHz): 168.65, 131.70, 129.82, 125.78, 121.61,120.14, 114.64, 113.17, 112.90, 20.93.
References:
Liu, Chunchen;Xu, Wenzhan;Xue, Qifan;Cai, Ping;Ying, Lei;Huang, Fei;Cao, Yong [Dyes and Pigments,2016,vol. 125,p. 54 - 63]
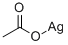
563-63-3
283 suppliers
$7.00/1g

372077-73-1
15 suppliers
inquiry

114306-17-1
41 suppliers
$125.00/100mg
52415-29-9
417 suppliers
$9.00/1g

114306-17-1
41 suppliers
$125.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

114306-17-1
41 suppliers
$125.00/100mg
116436-10-3
27 suppliers
$75.00/100mg

114306-17-1
41 suppliers
$125.00/100mg