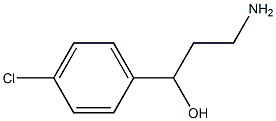
3-amino-1-(4-chlorophenyl)propan-1-ol synthesis
- Product Name:3-amino-1-(4-chlorophenyl)propan-1-ol
- CAS Number:46051-56-3
- Molecular formula:C9H12ClNO
- Molecular Weight:185.65
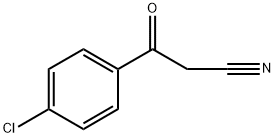
4640-66-8
251 suppliers
$6.00/1g

46051-56-3
7 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 3-(4-chlorophenyl)-3-oxopropanenitrilewith lithium aluminium hydride in tetrahydrofuran at 0;Reflux;
Stage #2: with lithium hydroxide monohydrate in tetrahydrofuran;
Steps:
19.1
Step 1: To an ice cooled solution of LAH (1.0 M solution in THF, 54 niL, 54 mmol) was added dropwise a solution of 3-(4-chlorophenyl)-3-oxopropanenitrile (3.20 g, 17.8 mmol) in THF (20 mL). The mixture was allowed to react at about 0 0C for lhourfollowed by reflux overnight. The solution was cooled to about 0 0C and the excess of LAH was quenched with water (2.0 mL) followed by 15% aqueous NaOH solution (2.0 mL) and water (6.0 mL). The mixture was diluted with Et2O (100 mL) and stirred at about room temperature for about 10 min. The mixture was filtered and the filtrate evaporated in vacuo. The residue was dissolved in DCM (35 mL). Et3N (3.7 mL, 20 mmol) was added followed by a solution of BoC2O (2.7 g, 27 mmol) in DCM (10 mL). The reaction was allowed to stir at about room temperature overnight. The mixture was washed with 2N HCl solution, saturated aqueous NaHCO3 solution, water and brine successively, dried and concentrated. The residue was purified by column chromatography (hexanes:EtOAc, 1 :1) to give tert-butyl 3-(4- chlorophenyl)-3-hydroxypropylcarbamate (3.0 g, 59%) as a solid. 1H NMR (CDCl3, 400 MHz) δ 7.30 (m, 4H), 4.85 (m, IH), 4.72 (m, IH), 3.48 (m, 2H), 1.81 (m, 2H), 1.46 (s, 9H).
References:
WO2009/89454,2009,A1 Location in patent:Page/Page column 101
1126-46-1
225 suppliers
$8.00/25g

46051-56-3
7 suppliers
inquiry