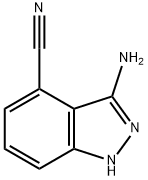
3-AMino-1H-indazole-4-carbonitrile synthesis
- Product Name:3-AMino-1H-indazole-4-carbonitrile
- CAS Number:1240518-54-0
- Molecular formula:C8H6N4
- Molecular Weight:158.16
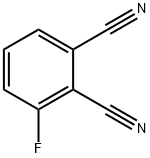
65610-13-1

1240518-54-0
General procedure for the synthesis of 3-amino-1H-indazole-4-carbonitrile from 3-fluorophthalonitrile: 3-fluoro-1,2-benzenedicarbonitrile (5 g, 34.2 mmol) was dissolved in ethanol (80 mL), and hydrazine monohydrate (4.98 mL, 103 mmol) was subsequently added to the solution. The reaction mixture was heated at 70 °C for overnight reaction. After completion of the reaction, the mixture was concentrated under vacuum and the residue was partitioned with ethyl acetate (200 mL) and water (200 mL). The organic layer was washed with water (100 mL) and the aqueous layer was then extracted with ethyl acetate (200 mL). All organic layers were combined and dried over anhydrous magnesium sulfate, followed by evaporation of the solvent under vacuum to give the title compound 3-amino-1H-indazole-4-carbonitrile (4.7 g, 87% yield) as a yellow solid.LCMS (System A) retention time (RT) = 0.53 min, electrospray positive ion mode (ES+ve) m/z 159 ([M+H]+).

65610-13-1
103 suppliers
$7.00/250mg

1240518-54-0
41 suppliers
inquiry
Yield:1240518-54-0 87%
Reaction Conditions:
with hydrazine hydrate in ethanol at 70;
Steps:
62
Intermediate 623-Amino-1H-indazole-4-carbonitrile; 3-Fluoro-1,2-benzenedicarbonitrile (APIN) (5 g, 34.2 mmol) was dissolved in ethanol (80 mL) and hydrazine monohydrate (4.98 mL, 103 mmol) was added to the solution. The reaction was heated at 70° C. overnight. The reaction mixture was concentrated in vacuo and the residue was partitioned between ethyl acetate (200 mL) and water (200 mL). The organic layer was washed with water (100 mL). The aqueous layers were extracted with ethyl acetate (200 mL). Organic layers were combined, dried over anhydrous magnesium sulfate and evaporated in vacuo to give the title compound (4.7 g, 87%) as a yellow solid. LCMS (System A) RT=0.53 min, ES+ve m/z 159 (M+H)+.
References:
US2010/216860,2010,A1 Location in patent:Page/Page column 32