
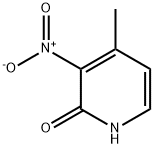
3-AMINO-2-HYDROXY-4-PICOLINE synthesis
- Product Name:3-AMINO-2-HYDROXY-4-PICOLINE
- CAS Number:33252-54-9
- Molecular formula:C6H8N2O
- Molecular Weight:124.14
21901-18-8
245 suppliers
$25.00/5g

33252-54-9
23 suppliers
inquiry
Yield:33252-54-9 99%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol at 20; under 672.31 Torr; for 18 h;
Steps:
95(1) Example 95 2-METHYL-4- [2- (4-TRIFLUOROMETHYLPHENYL) oxazolo [5, 4- B] PYRIDINE-7-YLMETHYLSULFANYL] PHENOXYACETIC Acid Step 1 3-Amino-4-methylpyridin-2-ol
Shake a suspension of commercially available 2-hydroxy-4- methoxy-3-nitrophenol (5 g, 32.4 mmol) and 10% palladium on carbon (300 mg) in ethanol (170 mL) at room temperature under hydrogen (13 psi) in a Parr bottle for 18 h. Filter the mixture through a short plug of celite, eluting with ethanol, and remove the solvents under reduced pressure to afford 2-amino-4-methylpyridin-2-ol (Step 1) as an off-white solid (4.16 g, >99%): 1H NMR (CDC13) B 2.00 (s, 3H), 4.00 (br s, 2H), 6.00 (d, 1H), 6.70 (d, 1H).
References:
WO2004/63155,2004,A1 Location in patent:Page 213-214