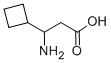
3-AMINO-3-CYCLOBUTYL-PROPIONIC ACID synthesis
- Product Name:3-AMINO-3-CYCLOBUTYL-PROPIONIC ACID
- CAS Number:887584-53-4
- Molecular formula:C7H13NO2
- Molecular Weight:143.18
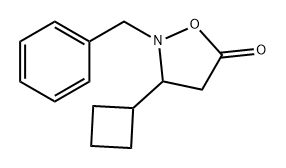
1422051-92-0
0 suppliers
inquiry

887584-53-4
14 suppliers
inquiry
Yield:887584-53-4 340 mg
Reaction Conditions:
Stage #1: 2-benzyl-3-cyclobutylisoxazolidin-5-onewith hydrogen;palladium(II) hydroxide in ethanol; under 2585.81 Torr; for 4 h;Inert atmosphere;
Stage #2: with palladium 10% on activated carbon;hydrogen in methanol; under 2585.81 Torr;
Steps:
1 Formation of (+/-)-3-amino-3-cyclobutylpropanoic acid (48a)
Formation of (+/-)-3-amino-3-cyclobutylpropanoic acid (48a)Dihydroxypalladium (0.252 g, 1.794 mmol) was charged into a flask and flushed with nitrogen. Ethanol (30 mL) was added followed by a solution of 2-benzyl-3-cyclobutyl- isoxazolidin-5-one, 47a, (0.834 g, 3.605 mmol) in approximately 90 mL of ethanol. The reaction mixture was subjected to 50 psi of hydrogen for 4 hours. The pressure was vented and the catalyst was filtered off. All volatiles were removed at reduced pressure. 1H NMR shows the presence of starting material, 47a. The mixture was dissolved in approximately 100 mL of MeOH and added to 83 mg of 10%Pd/C that had been wet with 20 mL of MeOH. The mixture was subjected to 50 psi of H2 overnight. The pressure was vented and the catalyst was filtered off. All volatiles were removed at reduced pressure to afford 340 mg of product. The resulting crude residue was used without further purification: 1H NMR (400 MHz, 6-DMSO) δ 3.06 - 2.83 (m, 1H), 2.28 (ddd, J= 23.7, 11.8, 7.7 Hz, 1H), 2.19 - 1.99 (m, 2H), 1.99 - 1.56 (m, 6H).
References:
WO2013/19828,2013,A1 Location in patent:Page/Page column 74-75

100144-52-3
10 suppliers
$175.00/50mg

887584-53-4
14 suppliers
inquiry
4415-82-1
306 suppliers
$6.00/1g

887584-53-4
14 suppliers
inquiry
2987-17-9
146 suppliers
$10.00/250mg

887584-53-4
14 suppliers
inquiry