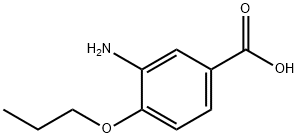
3-amino-4-propoxybenzoic acid synthesis
- Product Name:3-amino-4-propoxybenzoic acid
- CAS Number:59691-15-5
- Molecular formula:C10H13NO3
- Molecular Weight:195.22
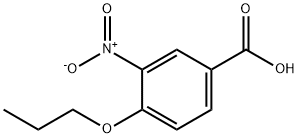
35288-44-9
60 suppliers
inquiry

59691-15-5
32 suppliers
inquiry
Yield:59691-15-5 97%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol at 20; for 1 h;
Steps:
46.C
A mixture of the product of Step B (0.38 g; 1.69 mmol) and 10% Pd/C (0.2 g) in EtOH (15 ml) was vigorously stirred for 1 h at room temperature under H2 (balloon). The catalyst was removed by filtration through Celite pad, washed with CH2CI2 (2 x 15 ml) and combined filtrates were evaporated to dryness under reduced pressure to give relevant aniline (0.32 g; 97%), which was suspended in H2O (3.3 ml) and t-BuOH (2.5 ml). To this (BoC)2O (0.72 g; 3.3 mmol) was added, followed by solid NaOH (0.07 g; 1.75 mmol) and the resulting mixture was stirred at ~ 6O0C for 3 h. The solvent were removed under reduced pressure and the residue was diluted to 5 ml with H2O, washed with Et2O (3 ml) and aqueous solution was acidified to pH ~ 3 with diluted HCI. The product was taken up with CH2CI2 (2 x 10 ml). The organic phase was dried over anhydrous MgSO4, filtered and filtrate evaporated to dryness under reduced pressure to give the title compound (0.17 g; 34%) as creamy solid. . 1 H-NMR (CDCI3) 1 .05 (tr, 3H, J = 9 Hz); 1 .54 (s, 1 H); 1.84 -1.91 (m, 2H); 4.02 - 4.07 (m, 2H); 6.86 (d, 1 H, J = 6 Hz); 7.02 (s, 1 H); 7.73 - 7.76 (m, 1 H); 8.76 (s, 1 H).
References:
WO2010/42998,2010,A1 Location in patent:Page/Page column 111