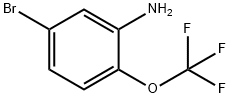
5-Bromo-2-(trifluoromethoxy)aniline synthesis
- Product Name:5-Bromo-2-(trifluoromethoxy)aniline
- CAS Number:886762-08-9
- Molecular formula:C7H5BrF3NO
- Molecular Weight:256.02
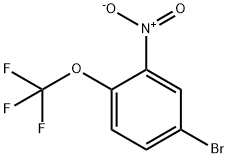
95668-20-5

886762-08-9
Synthesis of 5-bromo-2-(trifluoromethoxy)aniline (1): to a solution of 4-bromo-2-nitro-1-(trifluoromethoxy)benzene (2.0 g, 7.0 mmol) in acetic acid (10.0 mL) was added powdered iron (1.0 g, 17.9 mmol) batchwise at 0-10 °C. The reaction mixture was stirred at room temperature for 2 hours. Upon completion of the reaction, the acetic acid was removed by distillation and the residue was diluted with water and the pH was adjusted to alkaline with saturated sodium bicarbonate solution. Subsequently, the aqueous phase was extracted with ethyl acetate (2 x 50 mL). The organic layers were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give the brown colloidal solid product 1 (1.2 g, 70% yield).1H NMR (400 MHz, DMSO-d6): δ 5.68 (s, 2H), 6.65-6.66 (dd, J=2.4 Hz, 6.2 Hz, 1H), 6.95-6.96 (d, J=2.5 Hz. 1H), 7.01-7.01 (dd, J=1.4Hz, 8.6Hz, 1H). ms m/z (M+H): 256.3.

95668-20-5
120 suppliers
inquiry

886762-08-9
163 suppliers
$16.00/5 g
Yield:886762-08-9 70%
Reaction Conditions:
with iron;acetic acid at 0 - 20; for 2 h;
Steps:
58.1 5-bromo-2-(trifluoromethoxy)aniline (1):
5-bromo-2-(trifluoromethoxy)aniline (1): To a solution of 4-bromo-2-nitro-l- (trifluoromethoxy)benzene (2.0 g, 7.0 mmol) in acetic acid (10.0 mL) was added Fe -powder (1.0 g, 17.9 mmol) at 0-10 °C. The reaction mixture was allowed to stir at room temperature for 2 h. After completion of the reaction, acetic acid was distilled and the residue was diluted with water, basified with saturated sodium bicarbonate solution and extracted with ethyl acetate (2x50 mL). The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford 1 (1.2g, 70%) as a brown gummy solid. 1H NMR (400 MHz, DMSO- d6): δ 5.68 (s, 2H), 6.65-6.66 (dd, J = 2.4 Hz, 6.2 Hz, 1H), 6.95-6.96 (d, J = 2.5 Hz, 1H), 7.01- 7.01 (dd, J = 1.4 Hz, 8.6 Hz, 1H). MS m/z (M+H): 256.3.
References:
CELGENE AVILOMICS RESEARCH, INC.;ALEXANDER, Matthew David;MCDONALD, Joseph John;NI, Yike;NIU, Deqiang;PETTER, Russell C.;QIAO, Lixin;SINGH, Juswinder;WANG, Tao;ZHU, Zhendong WO2014/149164, 2014, A1 Location in patent:Paragraph 00758; 00892