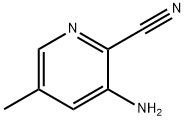
3-Amino-5-methylpyridine-2-carbonitrile synthesis
- Product Name:3-Amino-5-methylpyridine-2-carbonitrile
- CAS Number:1001635-30-8
- Molecular formula:C7H7N3
- Molecular Weight:133.15
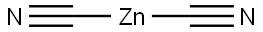
557-21-1
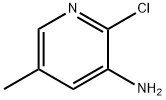
34552-13-1

1001635-30-8
Under argon protection, 1.0 g (7 mmol, 1 eq.) of 2-chloro-5-methylpyridin-3-amine was dissolved in 15 mL of anhydrous N,N-dimethylformamide (DMF) in a 20 mL reaction vial. Subsequently, 821 mg (7 mmol, 1 equiv) of zinc cyanide (Zn(CN)2) was added. After degassing the reaction system for 10 min, 405 mg (0.35 mmol, 0.05 eq.) of tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) was added. The reaction mixture was heated to 105 °C and the reaction was stirred at this temperature for 20 hours. After completion of the reaction, the mixture was filtered through diatomaceous earth and the filtrate was concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: ethyl acetate/petroleum ether, 2/8, v/v) to afford the white solid target product 3-amino-2-cyano-5-methylpyridine in 65% yield. The melting point of the product was 154 °C. Infrared spectrum (diamond ATR, cm^-1) ν: 3404, 2216, 1600, 1465, 1339, 1230, 858, 739; 1H NMR (400 MHz, CDCl3) δ: 2.34 (s, 3H, CH3), 4.37 (br s, 2H, NH2), 6.93 (d, 1H, J = 2.4 Hz. Harom), 7.93 (d, 1H, J = 2.4 Hz, Harom); 13C NMR (101 MHz, CDCl3) δ: 28.7 (CH3), 114.9 (CH), 116.3 (CN), 122.6 (CH), 138.6 (Cq), 142.2 (CH), 146.3 (Cq).
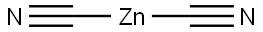
557-21-1
106 suppliers
$12.00/5g

34552-13-1
225 suppliers
$9.00/1g

1001635-30-8
92 suppliers
inquiry
Yield: 65%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0) in N,N-dimethyl-formamide at 105; for 20 h;Inert atmosphere;
Steps:
B.1.1 3-amino-5-methylpyridine-2-carbonitrile
Under an argon atmosphere, in a 20 mL vial, 1.0 g (7 mmol, 1 equiv.) of 2-chloro-5-methylpyridin-3-amine was dissolved in 15 mL of anhydrous DMF. 821 mg (7 mmol, 1 equiv.) of Zn(CN)2 were added. Next the solution was degassed for 10 minutes and 405 mg (0.35 mmol, 0.05 equiv.) of tetrakis(triphenylphosphino) palladium(0) were added. The mixture was heated to 105° C. for 20 hours. The reaction mixture was filtered on celite and evaporated in vacuo. The crude residue was then purified by chromatography column on silica gel under pressure (AE/EP 2/8) allowing isolation of a white solid with a yield of 65%. MP: 154° C. Infrared (Diamand ATR, cm-1) ν: 3404, 2216, 1600, 1465, 1339, 1230, 858, 739; 1H NMR (400 MHz, CDCl3) δ: 2.34 (m, 3H, CH3), 4.37 (bs, 2H, NH2), 6.93 (m, 1H, Harom), 7.93 (m, 1H, Harom); 13C NMR (101 MHz, CDCl3) δ: 28.7 (CH3), 114.9 (CH), 116.3 (CN), 122.6 (CH), 138.6 (Cq), 142.2 (CH), 146.3 (Cq)
References:
Routier, Sylvain;Benedetti, Hélène;Buron, Frédéric;Hiebel, Marie-Aude;Saurat, Thibault;Guillaumet, Gérald US2015/203489, 2015, A1 Location in patent:Paragraph 0660; 0661