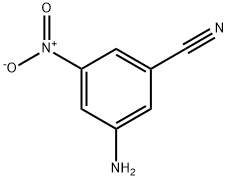
3-AMINO-5-NITROBENZONITRILE synthesis
- Product Name:3-AMINO-5-NITROBENZONITRILE
- CAS Number:10406-92-5
- Molecular formula:C7H5N3O2
- Molecular Weight:163.13
4110-35-4
138 suppliers
$19.00/1g

10406-92-5
12 suppliers
inquiry
Yield: 61%
Reaction Conditions:
with hydrogenchloride;iron in methanol at 20; for 0.5 h;
Steps:
Intermediate 7 IA: Preparation of 3 -amino-5-nitrobenzonitrile[00209] To a suspension of 3,5-dinitrobenzonitrile (4.5 g, 23.30 mmol) in MeOH (100 mL) was added concentrated HCl (15 mL), followed by iron powder (3.90 g, 69.9 mmol). The mixture was stirred at room temperature for 30 min. and then concentrated. The residue was treated with water and the resulting solid product was collected by filtration to give Intermediate 71A (2.3 g, 61%). HPLC: Rt = 1.245 min. (CHROMOLITH column 4.6 x 50 mm eluting with 10-90% aqueous methanol over 4 min. containing 0.1% TFA, 4 mL/min., monitoring at 220 nm). 1H NMR (500 MHz, DMSO-d6) δ ppm 7.68 (1 H, s), 7.64 (1 H, s), 7.24 (1 H, s), 6.33 (2 H, s).
References:
BRISTOL-MYERS SQUIBB COMPANY;FINK, Brian;CHEN, Libing;GAVAI, Ashvinikumar;HE, Liqi;KIM, Soong-Hoon;NATION, Andrew;ZHAO, Yufen;ZHANG, Litai WO2010/42699, 2010, A1 Location in patent:Page/Page column 104-105