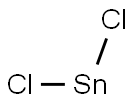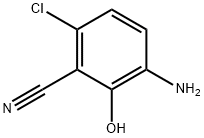
3-AMINO-6-CHLORO-2-HYDROXYBENZONITRILE synthesis
- Product Name:3-AMINO-6-CHLORO-2-HYDROXYBENZONITRILE
- CAS Number:201467-04-1
- Molecular formula:C7H5ClN2O
- Molecular Weight:168.58
Yield:201467-04-1 65%
Reaction Conditions:
in ethanol;
Steps:
15.c c)
c) Preparation of 2-amino-5-chloro6-cyanophenol A mixture of 2-nitro-5-chloro-6-cyanophenol (750 mg, 3.78 mmol) and tin (II) chloride (2.6 g, 11.4 mmol) in ethanol(50ml) is heated at 80° C. under argon. After 2 hours, the starting material has disappeared and the solution is allowed to cool down and then poured into ice. The pH is made slightly basic (pH7-8), by addition of solid NaOH, before being extracted with ethyl acetate. The organic phase was washed with brine, dried over MgSO4 and filtered. The solvent was evaporated and chromatography of the resulting solid on silica gel (4%MeOH/CH2Cl2) gave the desired product(410 mg, 65%). 1H NMR (CD30D): δ8 6.89 (d, 1H), 6.88 (d,1H).
References:
US6271261,2001,B1

201467-03-0
0 suppliers
inquiry

201467-04-1
5 suppliers
inquiry
89999-90-6
88 suppliers
$16.00/1g

201467-04-1
5 suppliers
inquiry
92161-40-5
4 suppliers
inquiry

201467-04-1
5 suppliers
inquiry