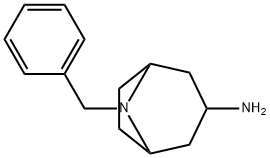
3-AMINO-8-BENZYL-8-AZABICYCLO[3.2.1]OCTANE synthesis
- Product Name:3-AMINO-8-BENZYL-8-AZABICYCLO[3.2.1]OCTANE
- CAS Number:96901-92-7
- Molecular formula:C14H20N2
- Molecular Weight:216.32
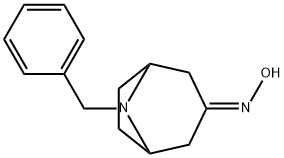
76272-34-9
![3-AMINO-8-BENZYL-8-AZABICYCLO[3.2.1]OCTANE](/CAS/GIF/96901-92-7.gif)
96901-92-7
The general procedure for the synthesis of 8-benzyl-8-azabicyclo[3.2.1]octan-3-amine from 8-benzyl-8-azabicyclo[3.2.1]octan-3-one oxime is as follows: these amines are key intermediates in the synthesis of compounds 4 and 20. First, 1-methylpiperazine or piperidine (1 mmol) and triethylamine (1.5 mmol, 0.21 mL) were sequentially added to a solution of 4-nitrobenzyl chloride (1 mmol) in anhydrous THF (3 mL). The reaction mixture was stirred at 70 °C overnight. Upon completion of the reaction, it was separated by extraction with dichloromethane and water. The organic phases were combined, dried over anhydrous Na2SO4 and subsequently concentrated under reduced pressure. The residue was purified by column chromatography (eluent ratio hexane/ethyl acetate 1:4) and the structure was confirmed by 1H NMR (see Supplementary Information). The purified product was dissolved in 10 mL of ethanol and PtO2 (0.01 g) was added under nitrogen protection. The hydrogenation reaction was carried out for 16 h at 50 psi hydrogen pressure using a Parr hydrogenation unit. At the end of the reaction, the catalyst was removed by filtration and the filtrate was concentrated under vacuum to give the target amine compound in quantitative yield.

76272-34-9
48 suppliers
$140.00/50mg
![3-AMINO-8-BENZYL-8-AZABICYCLO[3.2.1]OCTANE](/CAS/GIF/96901-92-7.gif)
96901-92-7
17 suppliers
inquiry
Yield:96901-92-7 80%
Reaction Conditions:
with sodium in pentan-1-ol; for 4 h;Heating / reflux;
References:
WO2004/54974,2004,A2 Location in patent:Page 55
![3-AMINO-8-BENZYL-8-AZABICYCLO[3.2.1]OCTANE](/CAS/GIF/96901-92-7.gif)
96901-92-7
17 suppliers
inquiry
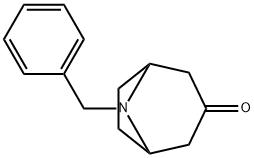
28957-72-4
211 suppliers
$28.00/1g
![3-AMINO-8-BENZYL-8-AZABICYCLO[3.2.1]OCTANE](/CAS/GIF/96901-92-7.gif)
96901-92-7
17 suppliers
inquiry

100-46-9
503 suppliers
$5.00/5G
![3-AMINO-8-BENZYL-8-AZABICYCLO[3.2.1]OCTANE](/CAS/GIF/96901-92-7.gif)
96901-92-7
17 suppliers
inquiry
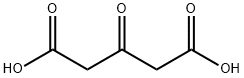
542-05-2
492 suppliers
$6.00/10g
![3-AMINO-8-BENZYL-8-AZABICYCLO[3.2.1]OCTANE](/CAS/GIF/96901-92-7.gif)
96901-92-7
17 suppliers
inquiry