3-AMINOBENZO[E][1,2,4]TRIAZIN-7-OL synthesis
- Product Name:3-AMINOBENZO[E][1,2,4]TRIAZIN-7-OL
- CAS Number:877874-01-6
- Molecular formula:C7H6N4O
- Molecular Weight:162.15
![3-AMINO-7-HYDROXYBENZO[E][1,2,4]TRIAZINE 1-OXIDE](/CAS2/GIF/157284-07-6.gif)
157284-07-6
3 suppliers
inquiry
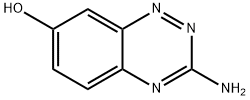
![3-AMINOBENZO[E][1,2,4]TRIAZIN-7-OL](/CAS2/GIF/877874-01-6.gif)
877874-01-6
10 suppliers
inquiry
Yield:877874-01-6 76%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol;N,N-dimethyl-formamide; for 3 h;
Steps:
I; II; 1; 3
4.6 g (25.82 mmol) of 3-amino-benzo[l,2,4]triazine-7-ol-l-oxide was dissolved in 200 mL of 1:1 mixture of dimethylformamide and methanol. 0.5 g of 10% Pd/C was added to this solution and H2 gas was bubbled through the solution for 3 hours. The progress of the reaction was monitored by TLC, using a 9:1 mixture of dichloromethane/methanol as an eluent and a UV lamp. The starting material is highly fluorescent under UV, while the product is not. When the reaction was complete, the resulting dark solution was filtered through a short pad of silica gel and solvent was removed in vacuum to produce a dirty-brown solid. 40 mL of ethyl acetate and 40 mL of methanol were added to the solid and the resulting suspension was heated to reflux for about 10 min. Then the suspension was allowed to cool down to ambient temperature. The solid was collected by filtration, washed with 40 mL of ethyl acetate, 40 mL of diethyl ether and dried in vacuum to yield 3.2 g of the product in a form of a greenish solid. Yield: 76%. XU NMR (DMSO-d6): 5 7.18 (s, 1 H), 7.36 (d, J= 2.6 Hz, 1 H), 7.40-7.42 (dd, Jj = 9.1 Hz, J2 = 2.6 Hz, 1 H), 7.45-7.46 (d, J= 9.1 Hz, 1H).
References:
WO2006/24034,2006,A1 Location in patent:Page/Page column 29-30; 64