
3-AMINOCINNAMIC ACID ETHYL ESTER synthesis
- Product Name:3-AMINOCINNAMIC ACID ETHYL ESTER
- CAS Number:125872-97-1
- Molecular formula:C11H13NO2
- Molecular Weight:191.23
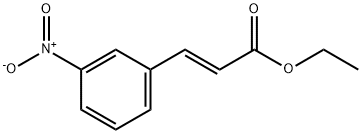
621-19-2
16 suppliers
inquiry

125872-97-1
37 suppliers
$72.50/2g
Yield:125872-97-1 100%
Reaction Conditions:
Stage #1: (E)-ethyl 3-(3-nitrophenyl)acrylatewith ethanol;water;tin(ll) chloride at 60; for 2 h;
Stage #2: with sodium hydrogencarbonate;Rochelle's salt in water;
Steps:
7.p.i
(p) 3- (3- [ (5-Phenyl-furan-2-carbonyl)-amino)-phenyl)-acrylic acid (61); (i) 3- (3-Amino-phenyl)-acrylic acid ethyl ester (60); 3- (3-Nitro-phenyl)-acrylic acid ethyl ester (500 mg, 2.3 mmol) was reduced using Method J to give the title compound. Yield: 430 mg, 100% ; LC-MS tr0. 92 min; MS (ES+) m/z 192 (M+H); J); The nitro derivative was dissolved in EtOH (5 vol) and SnCl2. 2H20 (50 eq) was added as a solid. The resulting solution was then stirred at 60°C for 2 h. After cooling to room temperature, a pre-mixed solution of saturated Rochelle's salt (10 vol) and saturated NaHC03 (10 vol) was added to the reaction mixture and the aqueous layer was extracted with EtOAc (3 x 20 vol). The combined organic layers were dried (MgSO4) and the solvent removed in vacuo to afford the product.
References:
WO2005/80367,2005,A1 Location in patent:Page/Page column 68; 115
59114-88-4
13 suppliers
inquiry

125872-97-1
37 suppliers
$72.50/2g
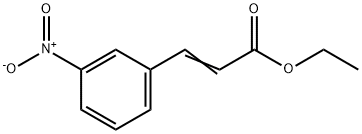
5396-71-4
76 suppliers
$28.60/50mg

125872-97-1
37 suppliers
$72.50/2g
626-01-7
262 suppliers
$8.00/5g
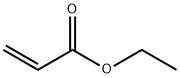
140-88-5
559 suppliers
$12.00/100ml

125872-97-1
37 suppliers
$72.50/2g
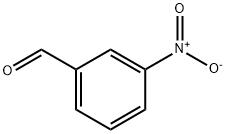
99-61-6
654 suppliers
$5.00/10g

125872-97-1
37 suppliers
$72.50/2g