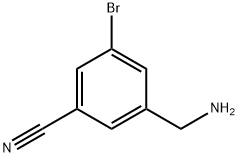
3-(aminomethyl)-5-bromobenzonitrile synthesis
- Product Name:3-(aminomethyl)-5-bromobenzonitrile
- CAS Number:1177558-50-7
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1177558-50-7
14 suppliers
inquiry
Yield: 55%
Reaction Conditions:
with hydrazine hydrate in ethanol;water for 3 h;Reflux;
Steps:
48 3-(Aminomethyl)-5-bromobenzonitrile
Example 48 3-(Aminomethyl)-5-bromobenzonitrile Hydrazine hydrate (85%, 1.31 g) was added to a suspension of 3-bromo-5-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]benzonitrile (3.50 g, 10.3 mmol) in EtOH (60 mL). The mixture was refluxed for 3 h. Then, at room temperature, 2 N HCl (20 mL) was added (pH=3), and the mixture was filtered and the solid was rinsed with water (20 mL*2). The filtrate was evaporated to about 50 mL and filtered again. After addition of NaHCO3 (pH=9), the filtrate was extracted with CH2Cl2 (50 mL*3). The combined extracts were washed with brine, dried over anhydrous sodium sulfate, and concentrated to give crude product. It was recrystallized from MeOH-Et2O yielding the product (1.40 g, 55%) as colorless fine needles. 1H NMR (400 MHz, D2O): δ 7.92 (m, 1H), 7.84 (m, 1H), 7.69 (m, 1H), 4.11 (s, 2H); 13C NMR (400 MHz, D2O): δ 137.1, 135.9, 135.8, 131.7, 123.0, 117.7, 113.8, 42.0; MS: m/z 209.0 (M+-HCl); HPLC: retention time: 9.313 min; purity: 98.4%.
References:
Glaxo Group Limited US2009/203657, 2009, A1 Location in patent:Page/Page column 54
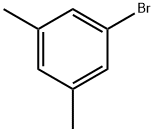
556-96-7
377 suppliers
$6.00/5g

1177558-50-7
14 suppliers
inquiry
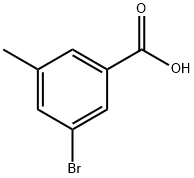
58530-13-5
125 suppliers
$8.00/1g

1177558-50-7
14 suppliers
inquiry
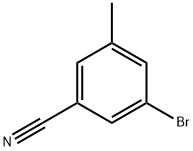
124289-21-0
115 suppliers
$8.00/100mg

1177558-50-7
14 suppliers
inquiry
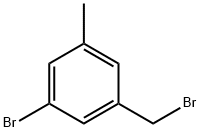
51719-69-8
27 suppliers
$87.00/250mg

1177558-50-7
14 suppliers
inquiry