
3-Benzofurancarboxamide synthesis
- Product Name:3-Benzofurancarboxamide
- CAS Number:959304-51-9
- Molecular formula:C9H7NO2
- Molecular Weight:161.16
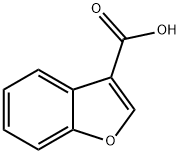
26537-68-8
94 suppliers
$37.00/100mg

959304-51-9
13 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:benzofurane-3-carboxylic acid with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 20; for 2 h;
Stage #2: with ammonia in dichloromethane;water
Steps:
4
EXAMPLE 4 N-(3-benzofuranylmethyl)-sulfamide (Compound #6) Benzofuran-3-carboxylic acid (1.91 g, 11.8 mmol) was suspended in anhydrous DCM (75 mL). Oxalyl chloride (2.0 M in DCM, 6.48 mL) and then one drop of dimethylformamide were added. The solution was stirred at room temperature for two hours, then ammonium hydroxide (concentrated, 10 mL) was added. The resulting mixture was diluted with water (100 mL) and extracted with DCM (3*100 mL). The extracts were concentrated to a gray solid and dissolved in anhydrous THF (100 mL). Lithium aluminum hydride (1.0 M in THF, 11.8 mL) was added. The mixture was stirred at room temperature for 16 hours. A minimal amount of saturated aqueous NaHCO3 and then MgSO4 were added. The mixture was filtered and then extracted with 1 N HCl. The aqueous extracts were adjusted to pH 14 with 3N NaOH and extracted with DCM. The organic extracts were dried with magnesium sulfate and concentrated to a colorless oil. The oil was dissolved in dioxane (50 mL) and sulfamide (3.7 g, 38 mmol) was added. The mixture was heated to reflux for 4 hours, cooled to room temperature, and concentrated. The resulting solid was chromatographed (5% methanol in DCM) to yield the title compound as a slightly yellow solid. 1H NMR (CD3OD): δ 7.53 (1H, d, J=5.7 Hz), 7.44 (1H, d, J=6.0 Hz), 7.16-7.26 (2H, m), 6.73 (1H, s), 4.35 (2H, s).
References:
Parker, Michael H.;Reitz, Allen B.;Maryanoff, Bruce E. US2006/47001, 2006, A1 Location in patent:Page/Page column 12-13