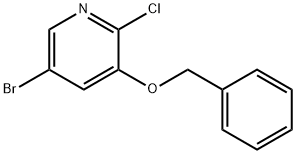
3-(Benzyloxy)-5-broMo-2-chloropyridine synthesis
- Product Name:3-(Benzyloxy)-5-broMo-2-chloropyridine
- CAS Number:891785-18-5
- Molecular formula:C12H9BrClNO
- Molecular Weight:298.56

552330-34-4

891785-18-5
Phosphorus trichloride (30 mL, 327.72 mmol) was added slowly and dropwise to an anhydrous dichloromethane solution of 3-benzyloxy-5-bromopyridine N-oxide (9 g, 32.13 mmol), and the reaction mixture was heated to reflux for 2 hours. Upon completion of the reaction, the solvent was evaporated under reduced pressure and the residue was carefully poured into saturated aqueous sodium carbonate solution. The precipitated solid was collected by filtration, washed with cold water and dried to give 5-bromo-2-3-benzyloxypyridine (9.10 g, 95% yield) as a beige solid. Melting point: 76-77 °C; IR (KBr, cm-1): 1547, 1415, 1266, 1071; 1H NMR (400 MHz, CDCl3): δ 5.34 (s, 2H, -OCH2Ph), 7.41-7.52 (m, 5H, Ph-H), 8.03 (d, J = 2.0 Hz, 1H, pyridine-H). 8.19 (d, J = 2.0 Hz, 1H, pyridine-H); 13C NMR (100 MHz, CDCl3): δ 70.8, 119.2, 124.6, 127.7, 128.3, 128.6, 135.5, 138.4, 140.8, 150.7; MS (ESI) m/z (relative abundance): 297.8 [(M + H)+, 72%], 299.8 [(M + H + 2)+, 100%], 301.8 [(M + H + 4)+, 27%]. Elemental analysis (C12H9BrClNO) calculated values: C, 48.27; H, 3.04; N, 4.69. measured values: C, 48.37; H, 3.05; N, 4.71.
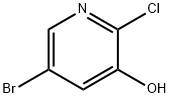
286946-77-8
157 suppliers
$13.00/100mg

100-39-0
429 suppliers
$10.00/10g

891785-18-5
28 suppliers
$36.00/100mg
Yield:891785-18-5 98%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 0 - 20; for 12 h;
Steps:
1.1 Step 1) Synthesis of A6
A mixture of 5-bromo-2-chloropyridine-3-ol (A5, 5.0 g, 2.40 mmol) and cesium carbonate (Cs2CO3, 12.0 g, 36.820 mmol) in dimethylformamide (DMF, 50 mL) was stirred at 0° C. To this stirred mixture was added benzyl bromide (3.40 ml, 28.57 mmol), slowly. The resulting solution was stirred at room temperature for 12 h. The mixture was diluted in ethyl acetate (EA). The organic layer was washed with distilled water, dried over Na2SO4, concentrated under reduced pressure to afford compound A6 (7.02 g) in 98.0% yield. ESI MS(m/e)=340 [M+CH3CN+H]+.
References:
US2022/298113,2022,A1 Location in patent:Paragraph 0047-0048

552330-34-4
0 suppliers
inquiry

891785-18-5
28 suppliers
$36.00/100mg
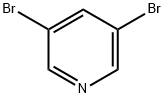
625-92-3
488 suppliers
$6.00/10g

891785-18-5
28 suppliers
$36.00/100mg
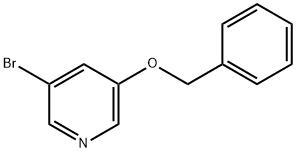
130722-95-1
115 suppliers
$7.00/250mg

891785-18-5
28 suppliers
$36.00/100mg

100-51-6
1434 suppliers
$5.00/100g

891785-18-5
28 suppliers
$36.00/100mg