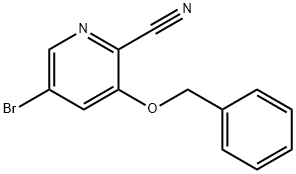
3-(BENZYLOXY)-5-BROMOPICOLINONITRILE synthesis
- Product Name:3-(BENZYLOXY)-5-BROMOPICOLINONITRILE
- CAS Number:1393534-88-7
- Molecular formula:C13H9BrN2O
- Molecular Weight:289.13
573675-25-9
268 suppliers
$13.00/5g

100-51-6
1370 suppliers
$5.00/100g

1393534-88-7
7 suppliers
inquiry
Yield:1393534-88-7 87.3%
Reaction Conditions:
Stage #1: benzyl alcoholwith sodium hydride in tetrahydrofuran at 0 - 35; for 1.5 h;Inert atmosphere;
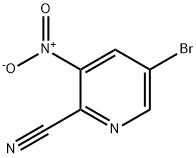
Stage #2: 5-bromo-3-nitropyridine-2-carbonitrile in tetrahydrofuran at 20; for 24 h;Inert atmosphere;Temperature;
Steps:
3-(Benzyloxy)-5-bromopicolinonitrile (6).
A mixture of THF (1.8L) and benzyl alcohol (59mL, 114mmol) was cooled to 0°C. NaH was added slowly to the mixture at 0°C. After addition, the mixture was stirred at room temperature for 1h and 35°C for another 0.5h. The resultant solution was added to the solution of 5-bromo-3-nitropicolinonitrile (5, 100g, 438mmol) in THF (1.3L) and the mixture was stirred at room temperature for 24h. The reaction mixture was quenched with H2O (200mL). After quenched, the mixture was concentrated to a volume of about 200mL. Dichloromethane (1.5L) was added, and the higher aqueous phase was removed. The organic phase was wash with saturated aqueous NaHCO3 (1.0L×1), water (1.0L×3), saturated aqueous NaCl (1.0L×1). The organic phase was concentrated, and dried under vacuum at 55°C to give 6 (110.0g, 87.3% yield, 99.5% purity by HPLC) as a brown solid.
References:
Lei, Yonghua;Hu, Tianhan;Hu, Mingyang;Yang, Li;Wu, Yue;Xiang, Hua;Zhang, Lianshan;Shen, Lingjia;You, Qidong;Zhang, Xiaojin [Tetrahedron Letters,2015,vol. 56,# 35,art. no. 46502,p. 5017 - 5019]