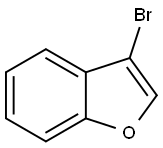
3-BROMO-1-BENZOFURAN synthesis
- Product Name:3-BROMO-1-BENZOFURAN
- CAS Number:59214-70-9
- Molecular formula:C8H5BrO
- Molecular Weight:197.03
![2,3-dibromo-2,3-dihydro-benzo[b]furan](/CAS/20180703/GIF/109210-15-3.gif)
109210-15-3

59214-70-9
The general procedure for the synthesis of 3-bromo-1-benzofuran from the compound (CAS: 109210-15-3) was as follows: first, potassium hydroxide pellets (9.3 g, 165 mmol) were dissolved in ethanol (40 mL) and the solution was cooled to 0 °C. Subsequently, 2,3-dibromo-2,3-dihydrobenzo[b]furan (23.0 g, 82.7 mmol) was dissolved in pre-cooled ethanol (90 mL) and the solution was added dropwise to the ethanol solution of potassium hydroxide at a constant temperature of 0 °C. After the dropwise addition, the reaction mixture was heated to reflux for 2 hours. After completion of the reaction, the mixture was concentrated under vacuum and then diluted by adding water (100 mL). The aqueous layer was extracted with ethyl acetate (3 × 100 mL). All organic phases were combined and washed with brine (100 mL), followed by drying with anhydrous magnesium sulfate and final concentration to give 14.7 g (90% yield) of the target product 3-bromo-1-benzofuran as an oil.
![2,3-dibromo-2,3-dihydro-benzo[b]furan](/CAS/20180703/GIF/109210-15-3.gif)
109210-15-3
1 suppliers
inquiry

59214-70-9
161 suppliers
$11.00/250mg
Yield:59214-70-9 90%
Reaction Conditions:
with potassium hydroxide in ethanol at 0; for 2 h;Heating / reflux;
Steps:
9.22
Potassium hydroxide pellets (9.3 g, 165 mmol) are dissolved in ethanol (40 mL) and cooled to 0° C. 2,3-Dibromo-2,3-dihydro-benzo[b]furan (23.0 g, 82.7 mmol) dissolved in ethanol (90 mL) is added dropwise hereto at a constant temperature of 0° C. After complete addition the mixture is heated to reflux for 2 hours. The mixture is concentrated in vacuo and water (100 mL) is added. The aqueous layer is extracted with ethyl acetate (3×100 mL). The combined organic fractions are washed with brine (100 mL), dried (MgSO4) and concentrated to furnish 14.7g (90%) of the wanted product as an oil.
References:
US2006/287386,2006,A1 Location in patent:Page/Page column 17
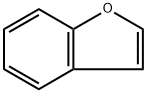
271-89-6
269 suppliers
$9.00/1g

59214-70-9
161 suppliers
$11.00/250mg
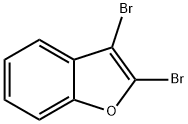
64150-61-4
27 suppliers
inquiry

271-89-6
269 suppliers
$9.00/1g

59214-70-9
161 suppliers
$11.00/250mg

64150-61-4
27 suppliers
inquiry

59214-70-9
161 suppliers
$11.00/250mg
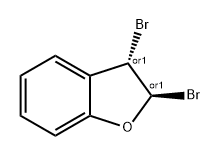
58863-52-8
0 suppliers
inquiry

59214-70-9
161 suppliers
$11.00/250mg