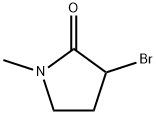
3-broMo-1-Methylpyrrolidin-2-one synthesis
- Product Name:3-broMo-1-Methylpyrrolidin-2-one
- CAS Number:33693-57-1
- Molecular formula:C5H8BrNO
- Molecular Weight:178.03
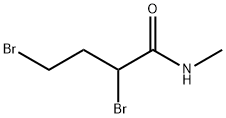
132996-62-4
2 suppliers
inquiry

33693-57-1
34 suppliers
$121.00/250 mg
Yield:33693-57-1 81%
Reaction Conditions:
with sodium hydride in tetrahydrofuran at 10 - 15;
Steps:
4
Intermediate 4: 3-Bromo-1-methyl-pyrrolidin-2-one Sodium hydride (60%, 1.2 g, mmol) was added portionwise to a solution of 2,4-dibromo-N-methyl-butanamide (7.8 g, 30 mmol) (Intermediate 5, CAS no. 33693-57-1) in THF (25 mL) under argon at 10-15° C. The mixture was added slowly to an ice-water mixture and extracted with DCM. The organic layer was separated and the aqueous layer re-extracted with DCM (2*10 mL). The combined organic layers were washed with brine (20 mL), dried (MgSO4) and evaporated to give a brown oil which was triturated with hexane and purified by column chromatography on silica eluding with 0-20% ethylacetate in DCM to afford the product (4.3 g, 81%). 1H NMR δ (400 MHz, CDCl3) 2.22-2.30 (m, 1H), 2.54 (sextet, 1H), 2.84 (s, 3H), 3.22-3.29 (dt, 1H), 3.46-3.54 (dt, 1H), 4.34 (d, 1H); m/z 178 (M+H)+
References:
AstraZeneca AB US2008/171734, 2008, A1 Location in patent:Page/Page column 37
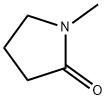
872-50-4
993 suppliers
$10.00/100ML

33693-57-1
34 suppliers
$121.00/250 mg
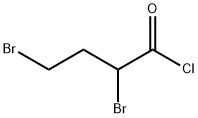
82820-87-9
63 suppliers
$35.35/250mgs:

33693-57-1
34 suppliers
$121.00/250 mg