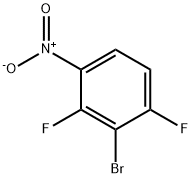
3-Bromo-2,4-difluoronitrobenzene 98% synthesis
- Product Name:3-Bromo-2,4-difluoronitrobenzene 98%
- CAS Number:103977-78-2
- Molecular formula:C6H2BrF2NO2
- Molecular Weight:237.99
64248-56-2

103977-78-2
The general procedure for the synthesis of 2-bromo-1,3-difluoro-4-nitrobenzene from 1-bromo-2,6-difluorobenzene was as follows: 2,6-difluorobromobenzene (125.5 g, 650 mmol) was dissolved in 98% sulfuric acid (250 mL) and the mixture was cooled in an ice-water bath. Subsequently, nitration reagent (100 mL) consisting of a 1:1 mixture of 98% sulfuric acid and fuming nitric acid was slowly added dropwise, and the rate of dropwise acceleration was controlled to ensure that the internal temperature of the reaction system did not exceed 35°C. The reaction was carried out in a controlled manner. After titration, the reaction mixture was stirred at room temperature for 3 hours. Upon completion of the reaction, the mixture was slowly poured into ice water and diluted with water to a final volume of 5 L. The precipitated solid product was collected by filtration, washed well with water, and then dried under vacuum in the presence of phosphorus pentoxide to afford a cream-colored solid of 2-bromo-1,3-difluoro-4-nitrobenzene (145 g, 94% yield). The product was characterized by 1H NMR (360 MHz, CDCl3): δ 7.15 (1H, m), 8.09-8.16 (1H, m).

64248-56-2
281 suppliers
$6.00/1g

103977-78-2
79 suppliers
inquiry
Yield: 97%
Reaction Conditions:
with nitric acid in sulfuric acid
Steps:
43.A 2,4-Difluoro-3-bromo-1-nitrobenzene
A. A solution of 1,3-difluoro-2-bromobenzene (23.5 g, 112 mmol) in concentrated sulfuric acid (48 ml) was stirred vigorously at ambient temperature. Concentrated nitric acid (70%, 8 ml) was added dropwise such that the internal temperature of the reaction mixture did not exceed 55° C. The reaction mixture was then stirred from 15 minutes and poured onto ice (300 ml). The aqueous mixture was extracted three times with methylene chloride. The combined organic layers were washed three times with a saturated sodium bicarbonate solution, dried over magnesium sulfate and filtered. Solvent was removed in vacuo to give a yellow solid. Recrystallization from isopropyl ether afforded a white solid (28.2 g, 97% yield): m.p. 50°-51.5° C. NMR (CDCl3, 60 MHz): 8.4-7.9 (m, 1H), 7.4-7.0 (m, 1H). Anal.: Calcd. for C6H2BrF2NO2: C, 30.28, H, 0.85, N, 5.89, Br, 33.57, F, 15.96. Found: C, 30.34, H, 0.98, N, 5.81, Br, 33.81, F, 16.04.
References:
Pfizer Inc. US4623650, 1986, A