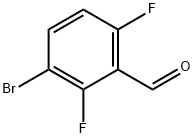
3-BROMO-2,6-DIFLUOROBENZALDEHYDE synthesis
- Product Name:3-BROMO-2,6-DIFLUOROBENZALDEHYDE
- CAS Number:398456-82-1
- Molecular formula:C7H3BrF2O
- Molecular Weight:221
348-57-2

68-12-2

398456-82-1
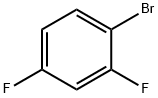
Lithium diisopropylammonium (2M THF solution, 13 mL, 26 mmol) was slowly added dropwise to a solution of anhydrous THF (80 mL) of 1-bromo-2,4-difluorobenzene (5.0 g, 25.9 mmol) at -78 °C, and the reaction was stirred for 1 h at -78 °C maintaining the temperature. Subsequently, N,N-dimethylformamide (2.0 g, 28 mmol) was added to the reaction system and stirring was continued at -78 °C for 30 min. After completion of the reaction, the reaction was quenched by adding water to the reaction mixture and extracted with ethyl acetate. The organic phases were combined, washed sequentially with 1N hydrochloric acid and water, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to remove the solvent. The residue was purified by silica gel column chromatography (eluent: ethyl acetate/hexane=1:9) to give 3.0 g of 3-bromo-2,6-difluorobenzaldehyde in 32% yield. The melting point of the product was 53-54 °C. 1H-NMR (CDCl3) δ: 7.00 (1H, dt, J = 9.3,1.8 Hz), 7.71-7.82 (1H, m), 10.3 (1H, s).

348-57-2
424 suppliers
$5.00/5g

398456-82-1
115 suppliers
$11.00/250mg
Yield:-
Reaction Conditions:
with lithium diisopropyl amide in tetrahydrofuran;N-methyl-acetamide;water
Steps:
106.a a)
a) 3-Bromo-2,6-difluorobenzaldehyde To a solution of 1-bromo-2,4-difluorobenzene (1.36 g, 7 mmol) in dry THF (20 mL) at -78° C. under nitrogen was added lithium diisopropylamide (2M in THF) (3.5 mL, 7 mmol) and stirred for 1h. Dimethylformamide (0.545 mL, 7 mmol) was added and stirred at -78° C. for 30 min. The reaction was quenched with acetic acid and extracted from water into dichloromethane. The organic extracts were washed with 1M hydrochloric acid, aqueous sodium hydrogen carbonate and brine, dried (MgSO4), filtered and evaporated in vacuo. The crude product was purified by flash chromatography on silica, eluding with ethyl acetate/hexane (3:17), to yield the title compound as a yellow solid.
References:
Agejas-Chicharro, Javier;Belen Bueno Melendo, Ana;Camp, Nicholas Paul;Gilmore, Jeremy;Jimenez-Aguado, Alma Maria;Lamas-Peteira, Carlos;Marcos-Llorente, Alicia;Mazanetz, Michael Philip;Montero Salgado, Carlos;Timms, Graham Henry;Williams, Andrew Caerwyn US2004/122001, 2004, A1