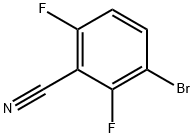
3-Bromo-2,6-difluorobenzonitrile synthesis
- Product Name:3-Bromo-2,6-difluorobenzonitrile
- CAS Number:1250444-23-5
- Molecular formula:C7H2BrF2N
- Molecular Weight:218
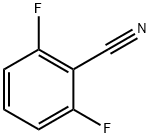
1897-52-5

1250444-23-5
General procedure for the synthesis of 3-bromo-2,6-difluorobenzonitrile from 2,6-difluorobenzonitrile: 600mL of 60% sulfuric acid was added to a 2L four-necked flask, and 139.0g (1.0 mol) of 2,6-difluorobenzonitrile was added while stirring. Under the protection of nitrogen, the reaction temperature was controlled below 35°C. 250.5 g of potassium bromate (1.5 mol) was added in 10 portions. The progress of the reaction was monitored by thin layer chromatography (TLC) and the reaction continued for 5-6 days until the raw material spot disappeared. After completion of the reaction, 800 mL of 20% ice brine solution was prepared and the reaction mixture was slowly poured into the ice brine. After the product settled, it was quickly filtered and washed with carbon tetrachloride (CCl4) to obtain the crude product, which was orange in color. The crude product was washed sequentially with 5% sodium bisulfite (NaHSO3) solution and water until it was neutral by saturated sodium carbonate (Na2CO3) solution. After drying with anhydrous sodium sulfate, it was filtered and evaporated by rotary evaporation in a water bath at 60°C to obtain a light yellow liquid. The liquid was cooled to room temperature and allowed to stand, precipitating a light yellow solid. Reduced-pressure distillation was carried out, the condensed steam was heated and the temperature of the oil bath was raised to 90°C. The vapor fraction at 58-62°C was collected and cooled to give a white solid product with a yield of 71%.

1897-52-5
539 suppliers
$7.00/10g

1250444-23-5
59 suppliers
inquiry
Yield: 71%
Reaction Conditions:
with potassium bromate;sulfuric acid at 35;Inert atmosphere;
Steps:
1.1 Preparation of 3-bromo-2,6-difluorophenyl nitrile
2 L four-necked flask, 600mL60% sulfuric acid was added, with stirring was added 2,6-difluorobenzonitrile 139.0g (1.0mol), in N2Under protection, control of the reaction temperature below 35°C, points added 250.5g of KBrO3 10 portions(1.5mol), TLC monitoring, the reaction 5-6 days, until starting material disappeared point, the reaction was stopped, the ice preparation 800mL20% saline solution, the reaction mixture was poured into iced brine.After settlement products, quickly suction filtration to give a crude orange product with CCl4The crude product was dissolved, respectively, with 5% NaHSO3Solution, saturated Na2CO3Solution was washed with water until the solution was neutral, dried over anhydrous sodium sulfate, the solution was filtered, 60 water bath rotary evaporation to give a pale yellow liquid was cooled to a standstill for some time as a pale yellow solid, distillation under reduced pressure, condensed steam heated, oil bath was raised to 90 deg.] C, taking the temperature of 58-62°C steam fraction, and cooled to obtain a white solid product, 71% yield.
References:
Tianjin Normal University;SONG, FANBO;ZHANG, ZHONGBIAO;LI, HUAYUN;TANG, HONGYING;WANG, ZHIQIANG;SONG, AIRU CN104004016, 2016, B Location in patent:Paragraph 0043; 0044; 0045

1897-52-5
539 suppliers
$7.00/10g
1541810-83-6
8 suppliers
inquiry

1250444-23-5
59 suppliers
inquiry