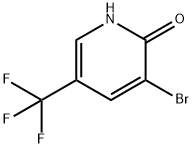
3-Bromo-2-hydroxy-5-(trifluoromethyl)pyridine synthesis
- Product Name:3-Bromo-2-hydroxy-5-(trifluoromethyl)pyridine
- CAS Number:76041-73-1
- Molecular formula:C6H3BrF3NO
- Molecular Weight:241.99
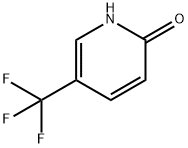
33252-63-0

76041-73-1
General procedure for the synthesis of 2-hydroxy-3-bromo-5-trifluoromethylpyridine from 2-hydroxy-5-trifluoromethylpyridine: to a solution of 5-(trifluoromethyl)pyridin-2-ol (10.52 g, 62 mmol) and sodium acetate (5.29 g, 64 mmol) in glacial acetic acid (38 mL) was added slowly with bromine (3.36 mL, 65 mmol) and the reaction was at room temperature. The reaction was carried out at room temperature. After observing the gradual transformation of the white turbid solution into a clarified brown solution, the reaction mixture was heated and refluxed at 80 °C for 2.5 h. After the reaction was completed, the mixture was cooled and refluxed for 2.5 h. The reaction was carried out at room temperature. Upon completion of the reaction, the mixture was cooled to room temperature and the solvent was subsequently removed by evaporation under reduced pressure. The residue was neutralized with saturated sodium bicarbonate solution to pH=8. The neutralized aqueous phase was extracted three times with ethyl acetate. The organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give the crude product 15.1 g (99.8% yield) as a white solid. The purity of the product was confirmed by LC-MS with a calculated value of [M+H]+ of C6H3BrF3NO of 241.9 and a measured value of 241.9/243.9.

33252-63-0
295 suppliers
$10.00/1g

76041-73-1
145 suppliers
$9.00/1g
Yield: 99.8%
Reaction Conditions:
Stage #1:2-hydroxy-5-(trifluoromethyl)pyridine with bromine;sodium acetate in acetic acid at 20 - 80; for 2.5 h;
Stage #2: with sodium hydrogencarbonate in water; pH=8
Steps:
5.A-1 4-[((1R,3S)-3-Isopropyl-3-{[3-(trifluoromethyl)-7,8-dihydro-1,6-naphthyridin-6(5H)-yl]carbonyl}cyclopentyl)amino]-1-(1,3-thiazol-2-yl)cyclohexanol
To a solution of 5-(trifluoromethyl)pyridin-2-ol (10.52 g, 62 mmol) and sodium acetate (5.29 g, 64 mmol) in glacial acetic acid (38 mL) was added bromine (3.36 mL, 65 mmol) at room temperature. The white cloudy solution slowly turned into a clear brown solution, which was heated at 80° C. for 2.5 h. The mixture was allowed to cool to room temperature and then evaporated under reduced pressure. The residue was neutralized with saturated NaHCO3 solution to pH=8. The resulting solution was extracted with EtOAc three times. The combined extracts were dried over MgSO4, filtered, and evaporated in vacuo to yield 15.1 g (99.8%) of the crude product (15.1 g, 98.8%) as a white solid. LC-MS calculated for C6H3BrF3NO: (M+H)+241.9; found 241.9/243.9. Step A-2
References:
Xue, Chu-Biao;Zheng, Changsheng;Cao, Ganfeng;Feng, Hao;Xia, Michael;Anand, Rajan;Glenn, Joseph;Metcalf, Brian W. US2005/267146, 2005, A1 Location in patent:Page/Page column 22-23

33252-63-0
295 suppliers
$10.00/1g

76041-73-1
145 suppliers
$9.00/1g