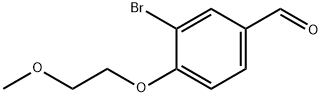
3-BroMo-4-(2-Methoxyethoxy)benzaldehyde synthesis
- Product Name:3-BroMo-4-(2-Methoxyethoxy)benzaldehyde
- CAS Number:915369-06-1
- Molecular formula:C10H11BrO3
- Molecular Weight:259.1
2973-78-6
224 suppliers
$14.00/5g

6482-24-2
296 suppliers
$9.00/1g

915369-06-1
7 suppliers
$395.00/10g
Yield:915369-06-1 70%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 50; for 24 h;
Steps:
24.1
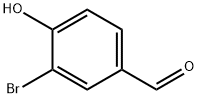
Example 24; 6-[3-Bromo-4-(2-methoxy-ethoxy)-benzylamino]-4-(3-chloro-4-fluoro-phenylamino)-quinoline-3-carbonitrile; Step 1: 3-bromo-4-hydroxybenzaldehyde (1 g, 4.97 mmol) was taken up in DMF (20 mL), then sodium hydride 60% (200 mg, 4.97 mmol) was added followed by 2-bromoethylmethylether (514 uL, 5.47 mmol) and heated at 50° C. for 24 hours. Then the mixture was diluted with ethyl acetate, washed with brine, dried over Mg2SO4. The residue was purified via flash column chromatography to obtain 3-bromo-4-(2-methoxy-ethoxy)-benzaldehyde (900 mg) in a 70% yield: 1H NMR (400 MHz, DMSO-D6) δ ppm 3.35 (s, 3 H) 3.71-3.75 (m, 2 H) 4.30-4.34 (m, 2 H) 7.33 (d, J=8.59 Hz, 1 H) 7.92 (dd, J=8.34, 2.02 Hz, 1 H) 8.11 (d, J=2.02 Hz, 1 H) 9.86 (s, 1 H).
References:
US2006/264460,2006,A1 Location in patent:Page/Page column 13-14